Figure 1.
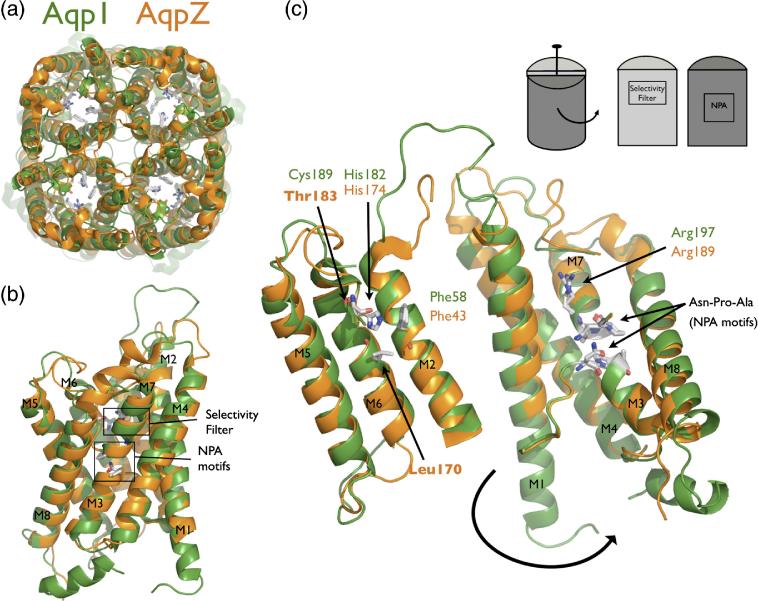
AqpZ is the bacterial homolog of AQP1. (A) Cartoon representation of the AqpZ (orange) and AQP1 (green) tetramers. Note the presence of the four monomer channels and the hypothetical channel down the tetramer axis. (B) Cartoon representation of the AqpZ and AQP1 monomers. Helices are labeled M1 through M8, and the selectivity filter and NPA motifs are designated with boxes. (C) Monomer opened up showing conservation of the water-selective motif. In this cartoon representation, the monomer is peeled open as shown in the inset schematic. The conserved selectivity filter and NPA motif are shown in sticks. Thr183 and Leu170 in AqpZ are the positions of cysteine mutants in this study. All molecular structure figures were made in Pymol (Delano Scientific).