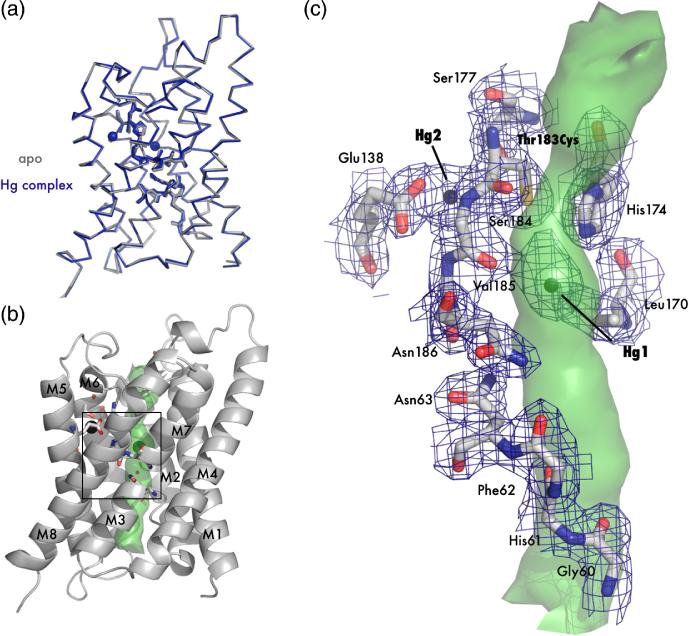
Figure 2.
Crystal structure of apo T183C and mercury bound T183C mutants. (A) Main chain overlay of the apo (gray) and Hg-complex (blue) with an RMSD (Cα) of 0.27 Å. Bound Hg2+ atoms are displayed as spheres with a van der Waals radius of 1.10 Å. (B) Cartoon representation of T183C. Transmembrane helices are labeled M1-M8 and the interior surface of the channel is drawn as a green surface. The black square denotes the area of interest depicted in panel C. (C) Structure of the blocked channel. Amino acids involved with water binding in AQPs are shown as sticks and with 2Fo-Fc electron density mapped contoured at 1.2σ drawn in blue. Mercury atoms are shown as spheres. In this orientation it can be seen that T183C-Hg1 sterically blocks the pore (green surface).