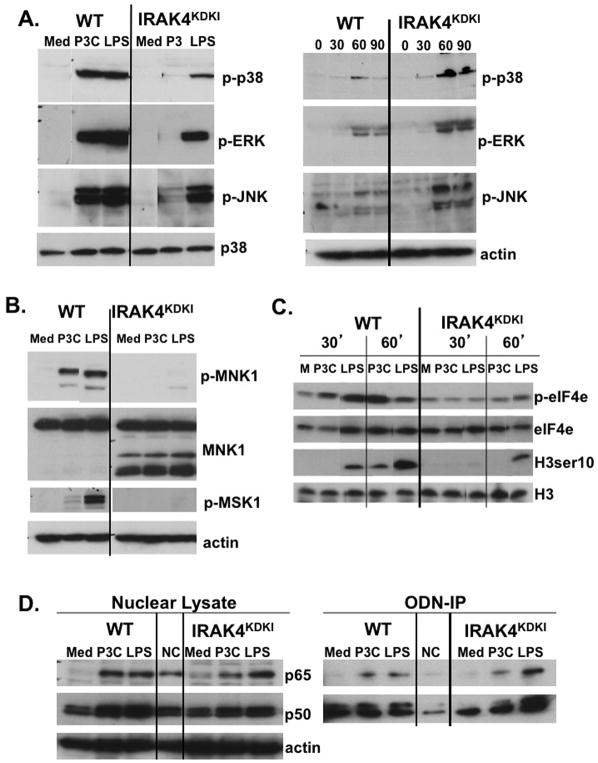
Figure 2. Activation of MAPKs is diminished in IRAK4KDKI macrophages in response to Pam3Cys and LPS.
(A) WT and IRAK4KDKI macrophages were treated with Pam3Cys (P3C; 1 μg/ml) or LPS (100 ng/ml) for 30 min (left panel) or Poly I:C (50 μg/ml) for 30, 60 or 90 min and cell lysates were analyzed by Western blot. Membranes were probed with anti-phospho-p38, anti-phospho-ERK1/2, and anti-phospho-JNK antibodies. Membranes were stripped and re-probed using anti-total p38 (left panel) or anti-actin (right panel) to ensure equal loading. (B) WT and IRAK4KDKI macrophages were stimulated with Pam3Cys (P3C; 1 μg/ml) or LPS (100 ng/ml) for 30 min and cell lysates were analyzed by Western blot using anti-phospho-MNK1, anti-total MNK1 and anti-phospho-MSK1 antibodies. Membranes were re-probed with anti-actin to ensure equal loading. (C) WT and IRAK4KDKI macrophages were treated Pam3Cys (P3C, 1 μg/ml) or LPS (100 ng/ml) for 30 and 60 min and analyzed by Western blot using anti-phospho-eIF4e antibody and re-probed using anti-total eIF4e antibody to ensure equal loading or anti-phospho-histone H3 ser 10 and re-probed using anti-total histone H3 to ensure equal loading. (D) WT and IRAK4KDKI macrophages were treated as in (A) for 1 h. Nuclear extracts were prepared and analyzed by Western blot using anti-p65, anti-p50 and anti-actin (loading control) antibodies (left panel). The same lysates were also incubated overnight with a synthetic biotinylated ODN containing the NF-κB consensus sequence or with a mutated sequence as a negative control (‘NC’). Biotinylated-ODNs were precipitated using streptavidin-conjugated agarose beads, protein was eluted with reducing sample buffer and analyzed by Western blot using anti-p65 or anti-p50 antibodies (right panel). All results are representative of at least three independent experiments.