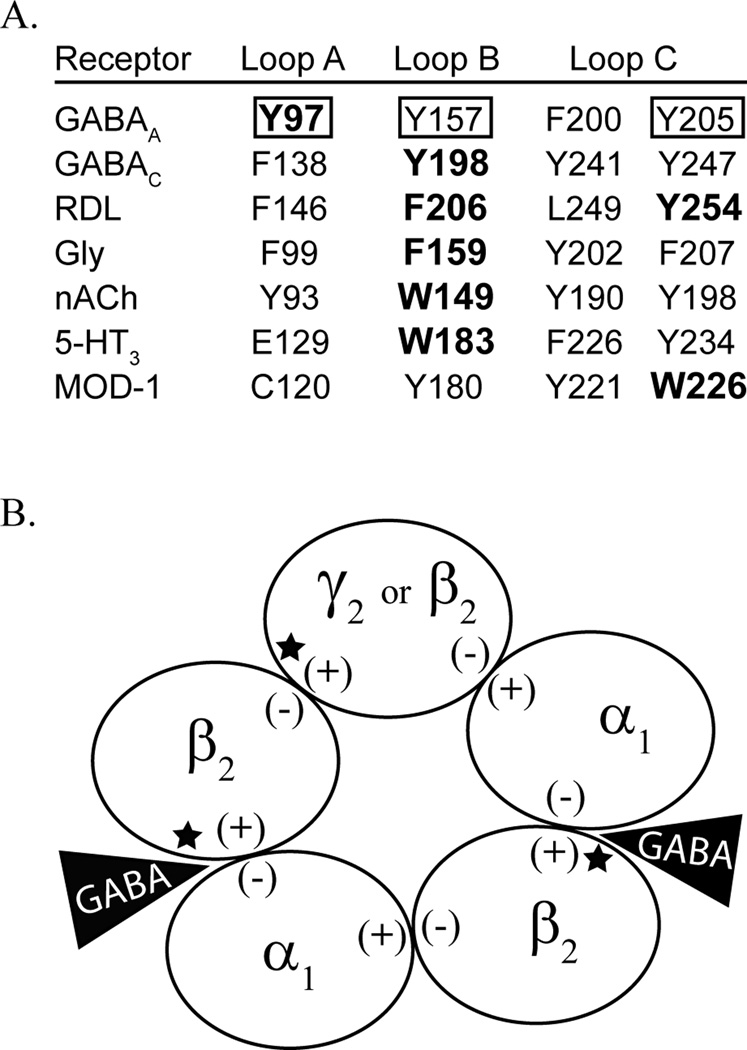
Figure 1.
The present study investigates tyrosine mutations of the β2 subunit. A) Alignment of the tyrosine residues in the binding pocket of the GABAA receptor, denoted by a box, with aromatic residues across the cys-loop family of receptors. Residues known to contribute to cation-π interactions are in bold. B) Depicted is a GABAA receptor that is comprised of 2α1, 2β2, and a fifth subunit that can be β2 or γ2. The (+) and (−) face of each subunit is labeled. The GABA binding sites, at the β/α interfaces, are indicated by a black triangle, and the locations of the mutations studied here are denoted by a star.