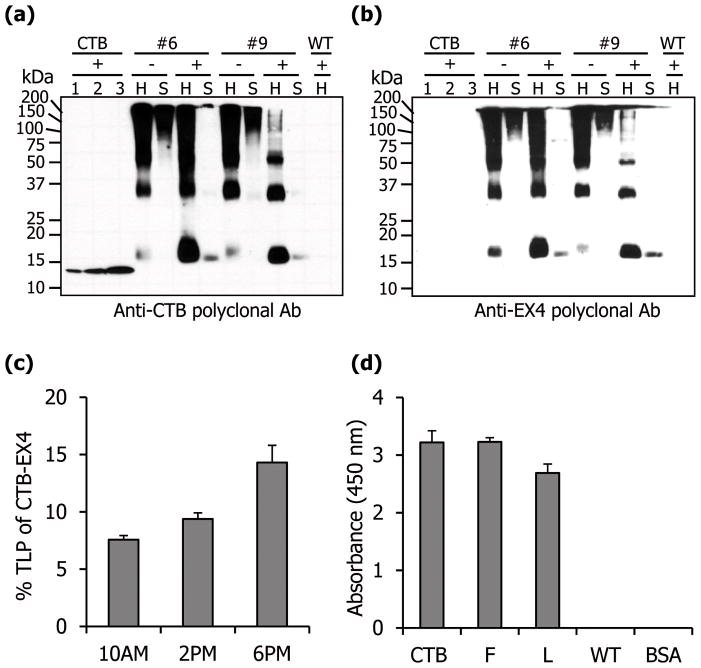
Figure 2.
Quantification and functional evaluation of CTB-EX4. Western blot analysis of total leaf homogenate (H, 5 μg) and soluble (S, 5 μg) protein probed with anti-CTB (a) or anti-EX4 (b) antibodies. Lane 1, 6.25; 2, 12.5; 3, 25 ng of purified cholera toxin B subunit; +, with DTT; −, without DTT; #6 and 9, CTB-EX4 transplastomic lines; WT, untransformed. (c) Percentage of CTB-EX4 in the total leaf protein from mature leaves at different harvesting time. (d) GM1 ELISA assay for evaluation of CTB-EX4 pentamer assembly. CTB, positive control (10 ng); F, fresh leaf; L, lyophilized CTB-EX4 transplastomic plant extracts (5 μg of total leaf protein), respectively; WT, untransformed total leaf protein (5 μg), BSA, negative control. Data shown are means ± SD of three independent experiments.