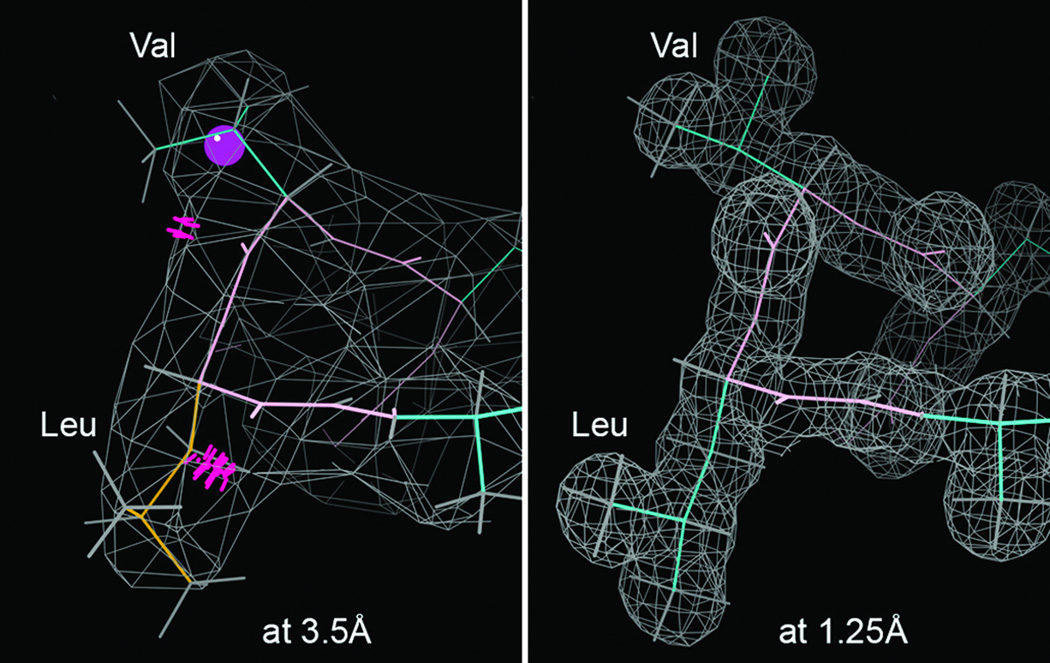
Figure 8.
Comparative information content and its consequences at high vs low resolution, for the same turn of helix in hemoglobin (β 108–111). At 1.25Å (2DN2; 79) in a well-ordered (low B-factor) region, each atom is observed and conformations are unambiguous. At 3.5Å (2QLS; 80) even in the best parts the electron density is smoothed out; in helix the backbone conformation can be inferred, but sidechains are too-small, uninformative blobs that are routinely misfit: here with a rotamer outlier (gold), a Cβ deviation (magenta ball), and 2 serious all-atom clashes (hotpink spikes), confirmed as incorrect by the high-resoluion structure.