Abstract
HIV integrase inhibitors such as raltegravir and elvitegravir halt HIV progression, but treatment-emergent resistance and cross-resistance have been observed. The nonnucleoside reverse transcriptase inhibitor etravirine (ETR) may be used in combination with integrase inhibitors in patients with drug resistance. This single-center, open-label, two-period, single-sequence crossover study evaluated the effects of ETR coadministration on the pharmacokinetic profile of S/GSK1265744, an investigational integrase inhibitor in phase 2 studies. Healthy subjects received 30 mg of S/GSK1265744 alone once daily for 10 days (period 1) and in combination with 200 mg of ETR twice daily for 14 days (period 2). Serial plasma samples for pharmacokinetic analyses were collected on day 10 during period 1 and on day 14 during period 2. All treatments were well tolerated. Etravirine had no effects on S/GSK1265744 geometric mean ratios of the area under the curve from time zero until the end of the dosing interval (1.01; 90% confidence interval [CI], 0.956 to 1.06), of the maximum observed plasma concentration (1.04; 90% CI, 0.987 to 1.09), or of the plasma concentration at the end of the dosing interval (0.999; 90% CI, 0.942 to 1.06). Etravirine pharmacokinetics (PK) parameters observed following coadministration with S/GSK1265744 were in the range of historical values reported for ETR alone in healthy subjects. These results indicate that 30 mg of S/GSK1265744 for 10 days as monotherapy followed by an additional 14 days in combination with ETR was well tolerated in healthy subjects and that no dose adjustment of S/GSK1265744 is required when it is coadministered with ETR.
INTRODUCTION
The emergence of the HIV integrase inhibitor (INI) class has expanded treatment and regimen sequencing options for patients with HIV. Although these agents have demonstrated excellent potency with favorable safety profiles, treatment-emergent resistance has been observed in patients failing regimens containing the INI raltegravir (RAL) (1, 2). Furthermore, cross-resistance between RAL and the investigational agent elvitegravir has also been reported (3). Therefore, there is a need for newer INIs with higher genetic barriers to resistance and a lower risk of cross-resistance. S/GSK1265744 is an INI selected for clinical development as a long-acting parenteral based on (i) a potential for a higher genetic barrier to resistance demonstrated through in vitro testing and (ii) a pharmacokinetic (PK) profile allowing low-dose, oral, once-daily dosing or parenteral once-monthly dosing (or longer) without the need for coadministration with a cytochrome P4503A (CYP3A) isozyme inhibitor such as ritonavir (4–6).
Etravirine (ETR) is a nonnucleoside reverse transcriptase inhibitor and an inducer of CYP3A enzymes (7) which may be involved in the clearance of some INIs. Since ETR has the potential to reduce concentrations of INIs, an assessment of the potential drug interaction between S/GSK1265744 and ETR was warranted. As ETR is predominantly metabolized by CYP enzymes and as S/GSK1265744 has no inductive or inhibitive effect on these enzymes, this study was designed to evaluate a one-way drug interaction effect of ETR on S/GSK1265744 PK in healthy subjects.
MATERIALS AND METHODS
Study design.
This single-center, open-label, two-period, single-sequence crossover study examined the PK of S/GSK1265744 alone (period 1) and in combination with ETR (period 2) in healthy subjects. Written informed consent was obtained from all subjects, and the protocols were approved by the institutional review board of the study site. Approximately 12 healthy subjects were planned for enrollment to provide data from 10 evaluable subjects. S/GSK1265744 at 30 mg was administered alone once daily (QD) for 10 days (period 1). Subjects then received 30 mg of S/GSK1265744 QD in combination with 200 mg of ETR twice daily (BID) for 14 days (period 2). All doses were given with a moderate-fat meal consistent with dosing recommendations for ETR, and there was no washout between periods. During both periods, S/GSK1265744 was administered in the morning. During period 2, the morning ETR dosing occurred with the administration of S/GSK1265744; the second daily dose of ETR was administered 12 h after the morning dose. All subjects remained in the clinical study site for the duration of the study and returned 7 to 14 days after the last dose for a follow-up visit.
Subjects were judged to be healthy by physical exam, medical history, and laboratory testing. Adult (i.e., 18 to 50 years old) males or females of nonchildbearing potential were enrolled. Subjects with a positive HIV or hepatitis C antibody result, a positive hepatitis B surface antigen result, a positive illicit drug or alcohol screen, and the use of any prescription or nonprescription drugs, including vitamins or herbal products, within 7 days or 5 half-lives (t1/2s) (whichever was longer) before the first dose were excluded.
For period 1, serial PK samples were collected on day 10 prior to dosing and then at 1, 2, 3, 4, 8, 12, and 24 h postdose. For period 2, serum samples for evaluation of predose PK concentrations were drawn in the morning on days 8, 11, 12, and 13, and serial PK sampling was repeated on day 14. Safety evaluations, including adverse-event (AE) assessments, vital signs, laboratory testing, and electrocardiograms, were performed at regular intervals throughout the studies. The severity of AEs and laboratory abnormalities were graded using the National Institute of Allergy and Infectious Diseases Division of AIDS grading table (8).
Assessments. (i) Bioanalytical methods.
After extraction from plasma by protein precipitation, serum S/GSK1265744 and ETR concentrations were determined using high-performance liquid chromatography using tandem mass spectrometry with TurboIonSpray and multiple reaction monitoring at GlaxoSmithKline (AB Sciex Analyst, version 1.4.2; Applied Biosystems, Framington, MA; SMS2000, version 2.1; GlaxoSmithKline, Research Triangle Park, NC). The validated calibration range was 10 to 10,000 ng/ml, and each analytical run included quality control (QC) samples at 40, 800, and 8,000 ng/ml. The validated calibration range for ETR was 5 to 4,500 ng/ml, and each analytical run included QC samples at 20, 500, and 3,600 ng/ml. Based on the analysis of these QC samples, the S/GSK1265744 bias ranged from −0.4% to 2.3% with a precision of <2.2%, and the ETR bias ranged from −2.2% to 4.2% with a precision of <2.6%.
(ii) Pharmacokinetic analysis.
A noncompartmental PK analysis of the concentration-time data was performed with WinNonlin (version 5.2; Pharsight Corp., St. Louis, MO). Plasma PK parameters for S/GSK1265744 and ETR were calculated using the actual recorded times for each treatment. Parameters that were determined include the area under the curve from time zero until the end of the dosing interval (AUC0-τ), the maximum observed plasma concentration (Cmax), the time to maximum observed plasma concentration (Tmax), the plasma concentration at the end of the dosing interval (Cτ), the minimum observed plasma concentration (Cmin), and the apparent terminal half-life (t1/2). The AUC0-τ was calculated by trapezoidal rule using the linear-up/log-down method.
Statistical analysis.
Statistical analysis was performed on the log-transformed PK parameters AUC0-τ, Cτ, and Cmax. Analysis of variance was performed using the SAS mixed-linear-models procedure (SAS Institute Inc., Cary, NC) to assess the effect of ETR on the PK of S/GSK1265744. Subject was fitted as a random effect, and treatment was fitted as a fixed effect in the model. The ratio of geometric least squares means and associated 90% confidence intervals (CIs) were estimated for the PK parameters of interest. S/GSK1265744 administered alone was considered to be the reference treatment, and S/GSK1265744 coadministered with ETR was considered to be the test treatment.
RESULTS
Demographics.
All 12 subjects who were enrolled completed the study. All subjects were male, with a median age of 29 years (range, 23 to 48 years) and a mean weight (± standard deviation) of 81.18 kg (± 8.161 kg). The majority of subjects (67%) were white.
Safety.
No deaths, serious AEs, or withdrawals due to AEs occurred during this study. One subject experienced a drug-related abnormality in clinical laboratory parameters (i.e., a grade 2 increase in blood amylase concentration during treatment with S/GSK1265744 plus ETR). No clinically significant vital signs or electrocardiogram (ECG) values were observed. The most commonly reported AEs were insomnia (four subjects receiving S/GSK1265744 plus ETR), nausea (two subjects receiving S/GSK1265744 and one subject receiving S/GSK1265744 plus ETR), and headache (two subjects receiving S/GSK1265744 plus ETR). No grade 3 or 4 AEs were reported.
Pharmacokinetics.
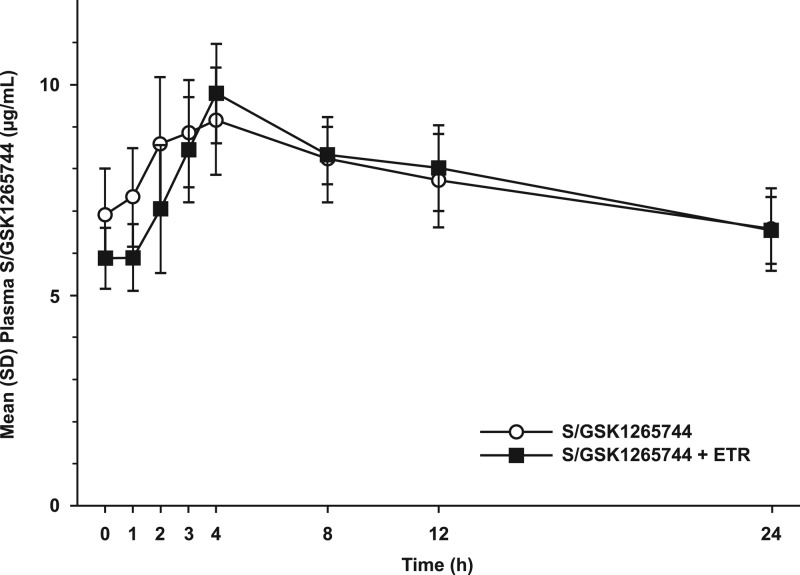
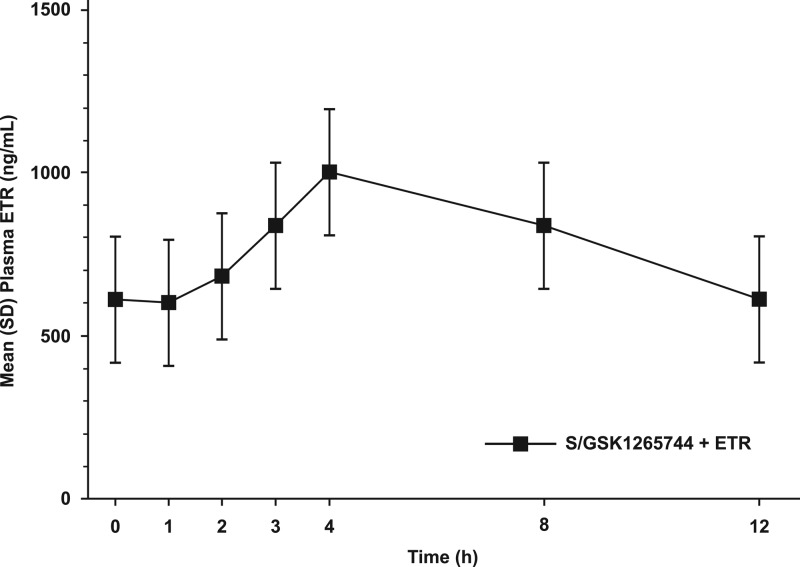
The steady-state mean plasma concentration-time profiles of S/GSK1265744 with or without coadministered ETR are shown in Fig. 1, and the steady-state mean plasma concentration-time profile of ETR when coadministered with S/GSK1265744 is shown in Fig. 2. Peak concentrations of S/GSK1265744 and ETR occurred within 3 to 4 h when they were administered alone or in combination, suggesting that S/GSK1265744 and ETR were readily absorbed. Pharmacokinetic parameters for S/GSK1265744 and ETR are provided in Table 1. Coadministration of S/GSK1265744 and ETR had no effect on S/GSK1265744 AUC0-τ, Cmax, or Cτ values (Table 2). Because PK sampling was conducted up to 24 h only, data were inadequate to estimate t1/2, which is not reported in the current study.
Fig 1.
Mean (SD) plasma concentration-time profile of 30 mg of S/GSK1265744 QD alone and when coadministered with 200 mg of etravirine (ETR) BID (n = 12).
Fig 2.
Mean (SD) plasma concentration-time profile of 200 mg of etravirine (ETR) BID when it is coadministered with 30 mg of S/GSK1265744 QD (n = 12).
Table 1.
Pharmacokinetic parameters of S/GSK1265744 and etravirine
| Treatment (n = 12) | Geometric mean value for the parameter (CVb [%])a |
||||||
|---|---|---|---|---|---|---|---|
| S/GSK1265744 |
ETR |
||||||
| AUC0-τ (μg · h/ml) | Cmax (μg/ml) | Cτ (μg/ml) | Median (range) Tmax (h) | AUC0-τ (ng · h/ml) | Cmax (ng/ml) | Cτ (μg/ml) | |
| S/GSK1265744 | 183 (13) | 9.47 (11) | 6.50 (14) | 3.04 (2.00–12.00) | |||
| S/GSK1265744 plus ETR | 184 (9) | 9.83 (11) | 6.49 (12) | 4.00 (2.00–8.00) | 9,176 (25) | 975 (24) | 597 (28) |
CVb, between-subject coefficient of variation.
Table 2.
Statistical comparisons of the pharmacokinetics of S/GSK1265744 alone or in combination with etravirine
| PK parametera | Plasma S/GSK1265744 + ETR vs S/GSK1265744 (GLS mean ratio [90% CI])b |
|---|---|
| AUC0-τ | 1.01 (0.956–1.06) |
| Cmax | 1.04 (0.987–1.09) |
| C0 | 0.854 (0.792–0.921) |
| Cτ | 0.999 (0.942–1.06) |
C0, drug concentration at the beginning of the dosing interval before administration of study drug for purposes of PK assessment (n = 12).
GLS, geometric least squares.
DISCUSSION
Etravirine is associated with numerous drug interactions that can affect coadministration with antiretrovirals or with nonantiretroviral drugs (9–11). Coadministration of the INI dolutegravir (DTG) and ETR reduced AUC0-τ and Cτ values for DTG by 71% and 88%, respectively, in healthy subjects (11). As DTG is metabolized via UDP-glucuronyltransferase 1A1 (UGT1A1; major metabolic pathway) and CYP3A4 (minor metabolic pathway), ETR induction of UGT1A1 has been suggested to be partially responsible for the reduction in DTG exposure (11). Raltegravir also is metabolized via UGT1A1, but coadministration of ETR and RAL in healthy subjects reduced the AUC from 0 to 12 h (AUC0–12) by 10% (geometric mean ratio, 0.90; 90% CI, 0.68 to 1.18) (12). Although the wide variation in the CIs suggests significant interindividual variability, this decrease was considered not to be clinically significant (12). However, a case series of four patients reporting virologic failure while receiving ETR and RAL showed significant decreases in trough concentrations of RAL (13). It is unclear if these case reports represented induction of UGT1A1, poor absorption, or the well-described large magnitude of intersubject PK variability with RAL (12–15). This study demonstrated that coadministration of S/GSK1265744 and ETR had no effect on the PK of S/GSK1265744. Although there are limited data on the metabolic pathway for S/GSK1265744, glucuronidation appears to be the primary pathway. The significant difference between the effect of ETR on DTG versus that on S/GSK1265744 may be due to the CYP component in the metabolism of DTG.
The PK parameters for ETR when it was coadministered with S/GSK1265744 were similar to historical values for ETR alone in healthy subjects, who were reported to have achieved a mean (SD) ETR AUC0-τ of 8,195 (2,428) ng · h/ml following administration of 200 mg of ETR BID (n = 39) (16). However, another study reported lower ETR concentrations than those reported here (12). Etravirine is metabolized by CYP2C9 and CYP2C19, each of which has been shown to be inhibited by S/GSK1265744 to a small extent in vitro. Raltegravir, which is not a substrate, an inhibitor, or an inducer of CYP enzymes, was shown to have no effect on ETR PK in a crossover study (12). However, between-study comparisons are complicated by potential differences in study design or assays, lack of study controls, PK variability, or variability of patient populations.
This study demonstrated that 30 mg of S/GSK1265744 QD for 10 days alone followed by 14 days in combination with 200 mg of ETR BID (i.e., 24 days of exposure) was well tolerated. Furthermore, coadministration of S/GSK1265744 and ETR had no effect on the PK of S/GSK1265744 in healthy subjects.
ACKNOWLEDGMENTS
Funding for this study was provided by Shionogi-ViiV Healthcare LLC.
We acknowledge the following individuals for editorial assistance during the development of the manuscript: Todd Parker, Patricia Zipfel, and Chris Lawrence.
All listed authors are employees of GlaxoSmithKline and meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Footnotes
Published ahead of print 31 October 2012
REFERENCES
- 1. Cooper DA, Steigbigel RT, Gatell JM, Rockstroh JK, Katlama C, Yeni P, Lazzarin A, Clotet B, Kumar PN, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Teppler H, Nguyen B-Y, for the BENCHMRK Study Teams 2008. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N. Engl. J. Med. 359:355–365 [DOI] [PubMed] [Google Scholar]
- 2. Mbisa JL, Martin SA, Cane PA. 2011. Patterns of resistance development with integrase inhibitors in HIV. Infect. Drug Resist. 4:65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeJesus E, Cohen C, Elion R, Ortiz R, Maroldo L, Franson S, Pesano R. 2007. First report of raltegravir (RAL, MK-0518) use after virologic rebound on elvitegravir (EVT, GS 9137), abstr TUPEB032. Abstr. 4th Int. AIDS Soc. Conf. HIV Pathog, Treatment Prev., Sydney, Australia [Google Scholar]
- 4. Min S, DeJesus E, McCurdy L, Richmond G, Torres J, Ford S, Chen S, Lou Y, Bomar M, Cyr T, Fujiwara T, St Clair M, Piscitelli S. 2009. Pharmacokinetics (PK) and safety in healthy and HIV-infected subjects and short-term antiviral efficacy of S/GSK1265744, a next generation once daily HIV integrase inhibitor, abstr H-1228. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. San Francisco, CA, 12 to 15 September 2009 [Google Scholar]
- 5. Underwood M, St Clair M, Johns B, Sato A, Fujiwara T, Spreen W. 2010. S/GSK1265744: a next generation integrase inhibitor (INI) with activity against raltegravir-resistant clinical isolates, abstr MOAA0103. Abstr. 18th Int. AIDS Conf., Vienna, Austria, 18 to 23 July 2010 [Google Scholar]
- 6. U.S. National Institutes of Health 2012. A study to investigate the safety, tolerability and pharmacokinetics of repeat dose administration of long-acting GSK1265744 and long-acting TMC278 intramuscular and subcutaneous injections in healthy adult subjects. Clinical trial NCT01593046. U.S. National Institutes of Health, Bethesda, MD: http://clinicaltrials.gov/ct2/show/NCT01593046?term=gsk1265744&rank=1 Accessed 8 June 2012 [Google Scholar]
- 7. Kakuda TN, Abel S, Davis J, Hamlin J, Schöller-Gyüre M, Mack R, Ndongo N, Petit W, Ridgway C, Sekar V, Tweedy S, Hoetelmans RMW. 2011. Pharmacokinetic interactions of maraviroc with darunavir-ritonavir, etravirine, and etravirine-darunavir-ritonavir in healthy volunteers: results of two drug interaction trials. Antimicrob. Agents Chemother. 55:2290–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute of Allergy and Infectious Diseases 2009. Division of AIDS table for grading severity of adult and pediatric adverse events. http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf National Institute of Allergy and Infectious Diseases, Bethesda, MD [Google Scholar]
- 9. Janssen Therapeutics 2011. Intelence package insert. Janssen Therapeutics, Titusville, NJ: http://www.intelence-info.com/sites/default/files/pdf/INTELENCE_Booklet_Package_Insert_hcp.pdf [Google Scholar]
- 10. Kakuda TN, Schöller-Gyüre M, Hoetelmans RMW. 2011. Pharmacokinetic interactions between etravirine and non-antiretroviral drugs. Clin. Pharmacokinet. 50:25–39 [DOI] [PubMed] [Google Scholar]
- 11. Song I, Borland J, Min S, Lou Y, Chen S, Patel P, Wajima T, Piscitelli SC. 2011. Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir. Antimicrob. Agents Chemother. 55:3517–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson MS, Kakuda TN, Hanley W, Miller J, Kost JT, Stoltz R, Wenning LA, Stone JA, Hoetelmans RMW, Wagner JA, Iwamoto M. 2008. Minimal pharmacokinetic interaction between the human immunodeficiency virus nonnucleoside reverse transcriptase inhibitor etravirine and the integrase inhibitor raltegravir in healthy subjects. Antimicrob. Agents Chemother. 52:4228–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ménard A, Solas C, Mokthari S, Bregigeon S, Drogoul M-P, Tamalet C, Lacarelle B, Martin IP. 2009. Etravirine–raltegravir, a marked interaction in HIV-1-infected patients: about four cases. AIDS 23:869–871 [DOI] [PubMed] [Google Scholar]
- 14. Cattaneo D, Gervasoni C, Meraviglia P, Landonio S, Fucile S, Cozzi V, Baldelli S, Pellegrini M, Galli M, Clementi E. 2012. Inter- and intra-patient variability of raltegravir pharmacokinetics in HIV-1-infected subjects. J. Antimicrob. Chemother. 67:460–464 [DOI] [PubMed] [Google Scholar]
- 15. Eron JJ, Jr, Rockstroh JK, Reynes J, Andrade-Villanueva J, Ramalho-Madruga JV, Bekker L-G, Young B, Katlama C, Gatell-Artigas JM, Arribas JR, Nelson M, Campbell H, Zhao J, Rodgers AJ, Rizk ML, Wenning L, Miller MD, Hazuda D, DiNubile MJ, Leavitt R, Isaacs R, Robertson MN, Sklar P, Nguyen B-Y. 2011. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non-inferiority trial. Lancet Infect. Dis. 11:907–915 [DOI] [PubMed] [Google Scholar]
- 16. Scholler-Gyure M, Kakuda TN, De Smedt G, Woodfall B, Lachaert R, Beets G, Peeters M, Hoetelmans R. 2007. Pharmacokinetics of TMC125 in once- and twice-daily regimens in HIV-1-negative volunteers, abstr A-1427. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007 [Google Scholar]