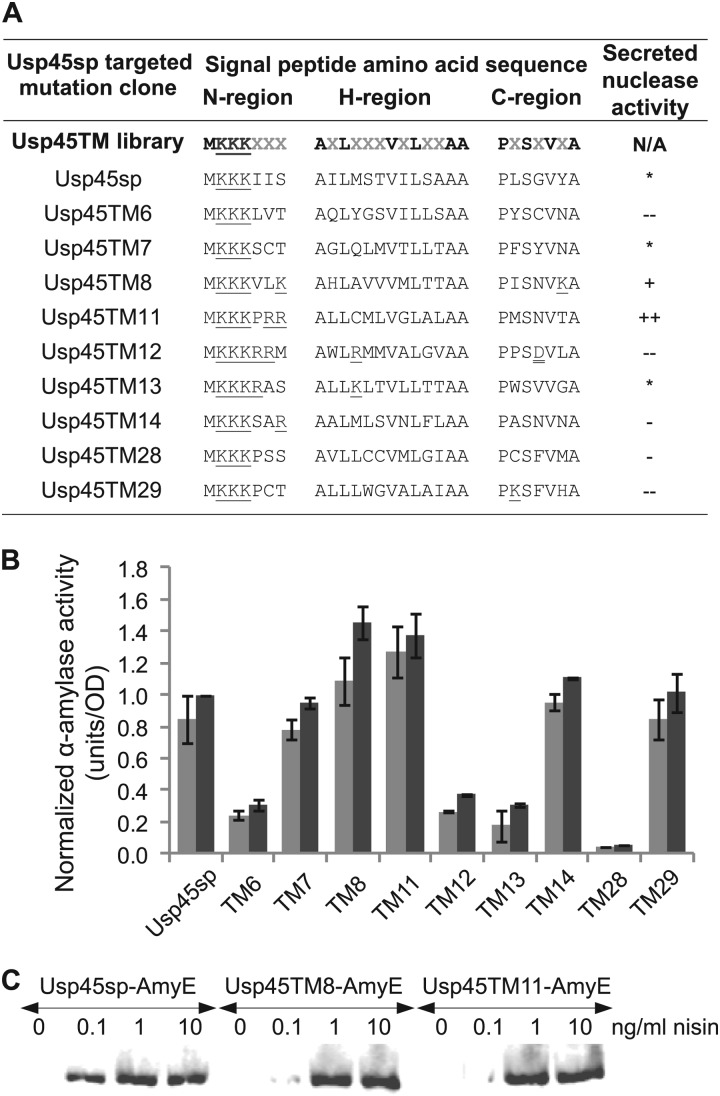
Fig 4.
Secretion efficiencies of clones selected from the targeted mutagenesis library of the Usp45 signal peptide. (A) Amino acid sequences and secreted nuclease activity for cultures induced at 10 ng/ml nisin. Amino acid mutation positions, indicated by gray Xs in the Usp45TM library sequence, were encoded by degenerate NNK nucleotides. *, −, −−, +, and ++ indicate secretion levels similar to and <10% lower, >10% lower, <10% higher, and >10% higher than Usp45sp levels, respectively. Single and double underlines indicate positively and negatively charged residues, respectively. Nucleotide sequences are given in Table S5 in the supplemental material. (B) α-Amylase activity of different clones normalized to the amount secreted by Usp45sp culture induced with 10 ng/ml nisin. Light gray bars represent induction at 1 ng/ml nisin, and dark gray bars represent induction at 10 ng/ml nisin. The data represent the means ± SEM of the results of two independent experiments. (C) Western blot showing secreted α-amylase in the supernatant detected by anti-RGS-His antibody. The different targeted mutations did not alter the size of the secreted protein, indicating that the signal peptide cleavage site had been maintained.