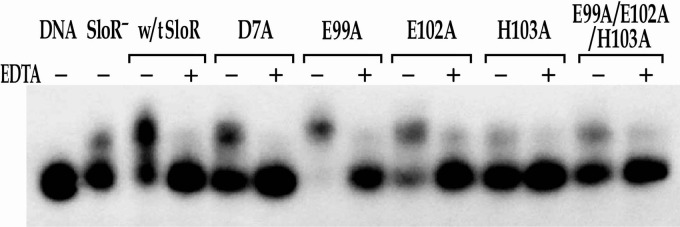
Fig 4.
Binding of wild-type (w/t) SloR and its metal ion-binding site 2 mutant variants to the sloABC promoter in EMSA. EMSAs were performed with a [γ-32P]ATP end-labeled 364-bp sloABC promoter-containing amplicon (the equivalent of 38 picograms) and whole-cell lysates containing 6.5 μg of total protein prepared from the GMS182 fusion strain and its derivatives. The shift in the lane without SloR (SloR−) is likely the result of nonspecific interactions between the target DNA and proteins present in the whole-cell lysate. This level of background was accounted for in subsequent quantitative analyses. When appropriate, EDTA was added at a final concentration of 15 mM. Reaction mixtures were run on 6% nondenaturing polyacrylamide gels and exposed for up to 48 h at −80°C in the presence of an intensifying screen. All of the metal ion-binding site 2 variants with the exception of SloR(E99A) were 30% to 90% compromised in their ability to bind DNA compared to wild-type SloR's binding, consistent with the predicted role for site 2 in activating DNA binding.