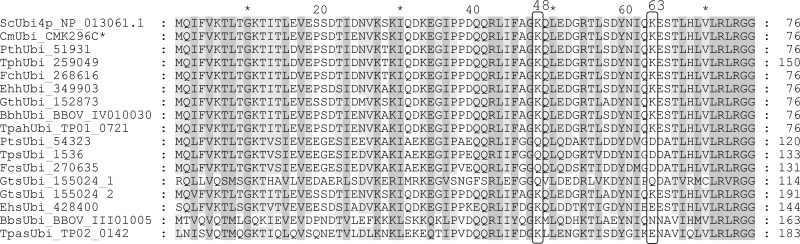
Fig 2.
Alignment of the ubiquitin domain of hUbi and sUbi sequences with the S. cerevisiae and C. merolae ubiquitins. The host ubiquitin sequences are derived from polyubiquitins consisting of multiple ubiquitin domains of the same sequence. Protein sequences of sUbi from P. tricornutum, F. cylindrus, and T. pseudonana share the same lysine mutations at positions 48 and 63. E. huxleyi, T. parva, and B. bovis only show an altered Lys63 position. Both ubiquitin domains of the F. cylindrus symbiont diubiquitin have identical protein sequences; in contrast, the diubiquitin of G. theta is depicted as two independent ubiquitin domains (GtsUbi_155024_1/2). (*, There is no polyubiquitin in C. merolae; therefore, a ubiquitin-ribosomal fusion protein was included in the alignment. For detailed information on the protein sequences, see File S1 in the supplemental material).