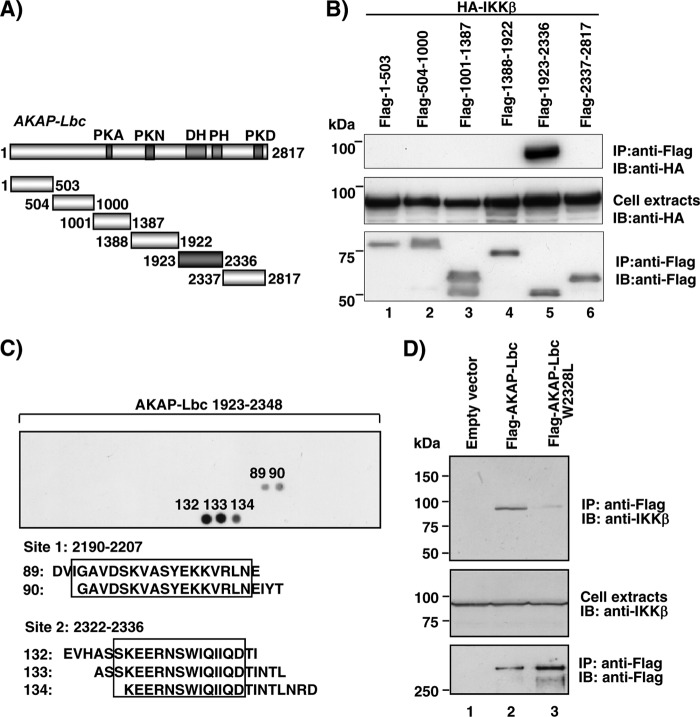
Fig 3.
Mapping of the IKKβ binding site(s) on AKAP-Lbc. (A) Schematic representation of the protein domain organization of AKAP-Lbc. The PKA binding domain (PKA), the PKN- and PKD-interacting region, and the Dbl (DH) and pleckstrin (PH) homology domains are shown. (B) HEK293 cells were transfected with HA-tagged IKKβ in combination with Flag-tagged fragments of AKAP-Lbc encompassing residues 1 to 503, 504 to 1000, 1001 to 1387, 1388 to 1922, 1923 to 2336, and 2337 to 2817. Cell lysates were subjected to immunoprecipitation with anti-Flag antibodies. Western blots of the immunoprecipitates and of the cell extracts were revealed using anti-HA polyclonal antibodies to detect HA-IKKβ (top and middle) or anti-Flag monoclonal antibodies to detect the Flag-tagged AKAP-Lbc fragments (bottom). (C) Peptide array analysis of the IKKβ binding region on AKAP-Lbc. The array was incubated with 10 nM S-tagged IKKβ-307-756 fragment. Solid-phase binding was assessed using HRP-conjugated S protein. The IKKβ binding peptides are numbered. (D) HEK293 cell lysates transfected with control vector or plasmids encoding Flag-AKAP-Lbc or Flag-AKAP-Lbc W2328L were immunoprecipitated using anti-Flag antibodies. Proteins in the extracts and immunoprecipitates were identified by immunoblotting using antibodies against IKKβ or the Flag tag, as indicated. Data are representative of three independent experiments.