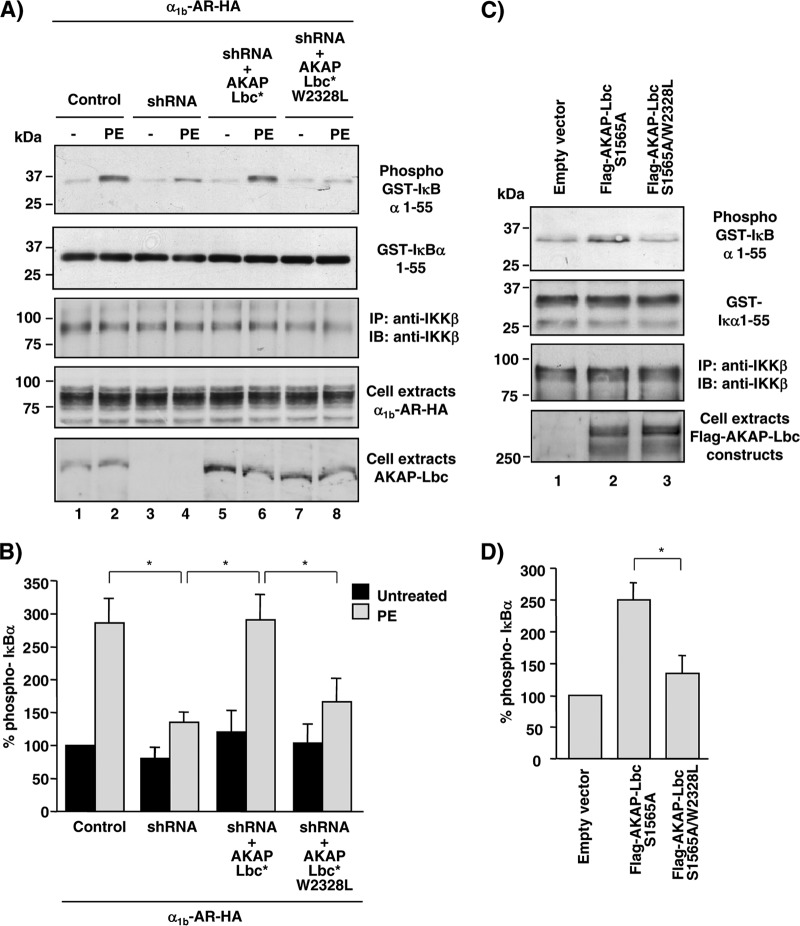
Fig 5.
Formation of the AKAP-Lbc/IKKβ complex is required for AKAP-Lbc-mediated IKKβ activation. (A) HEK293 cells were infected using control lentiviruses or lentiviruses carrying AKAP-Lbc shRNAs and subsequently transfected with either the vector encoding the HA-tagged α1b-AR alone or the vector encoding the HA-tagged α1b-AR together with the plasmids encoding the silencing resistant mutants of AKAP-Lbc (AKAP-Lbc*) or AKAP-Lbc W2328L (AKAP-Lbc* W2328L). After a 24-h serum starvation, cells were treated with phenylephrine (PE) for 15 min or left untreated. IKKβ immunocomplexes were isolated from cell extracts and incubated with GST-IκBα-1-55 and ATP. Phospho-GST-IκBα-1-55 was detected by immunoblotting using anti-phosphoserine 32/34-specific antibodies (top). (B) Quantitative analysis of phosphorylated GST-IκBα-1-55 was obtained by densitometry. The amount of phospho-GST-IκBα-1-55 was normalized to the total amount of GST-IκBα-1-55 and IKKβ. Results are expressed as means ± SE from 3 experiments. *, P < 0.05. The amounts of GST-IκBα-1-55, IKKβ, and Flag-AKAP-Lbc constructs and HA-α1b-AR were detected by Western blotting using specific antibodies as indicated. (C) HEK293 cells were transfected with the empty pFlag vector or the plasmids encoding Flag-AKAP-Lbc-S1565A or Flag-AKAP-Lbc-S1565A/W2328L. After a 24-h serum starvation, IKKβ immunocomplexes were isolated from cell extracts and used to phosphorylate purified GST-IκBα-1-55 as indicated for panel A. (D) Quantitative analysis of phosphorylated GST-IκBα-1-55 was obtained as indicated for panel B. Results are expressed as means ± SE from 4 experiments. *, P < 0.05.