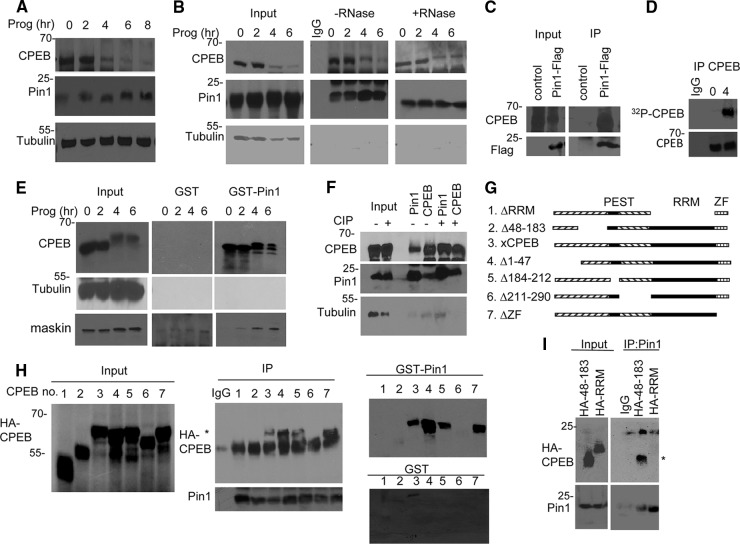
Fig 1.
CPEB and Pin1 interact in a phosphorylation-independent manner. (A) Western blots of CPEB and Pin1 during progesterone-induced oocyte maturation. Tubulin served as a loading control. (B) Extracts from oocytes exposed to progesterone for 0 to 6 h were subjected to Pin1 immunoprecipitation in the absence or presence of RNase A and Western blotting for Pin1, CPEB, and tubulin. The input represents 10% of the total lysate. (C) Oocytes were injected with mRNA encoding Flag-Pin1 and subjected to Flag immunoprecipitation and Western blot analysis for Flag and CPEB. Control refers to nonspecific IgG immunoprecipitation. (D) Oocytes were injected with [γ-32P]ATP, followed by treatment with progesterone for 0 or 4 h, immunoprecipitation of CPEB, and SDS-PAGE and autoradiography. (E) Extracts from oocytes exposed to progesterone for 0 to 6 h were applied to GST or GST-Pin1 columns, followed by Western blotting for CPEB, tubulin, and maskin. (F) Oocyte extracts were treated with calf intestinal alkaline phosphatase (CIP) prior to immunoprecipitation of Pin1 and CPEB and blotting for CPEB, Pin1, and tubulin. (G) Schematic of CPEB deletion mutants. (H) mRNA encoding HA-tagged CPEB deletion mutants (numbered in panel G) were injected into oocytes, followed by Pin1 immunoprecipitation and Western blotting for Pin1 and HA (center panel) or chromatography on a GST or GST-Pin1 column (right panels). The asterisk denotes the HA-CPEB proteins. (I) mRNA encoding HA-tagged CPEB proteins 48 to 183 and RRM were injected into oocytes, followed by Pin1 immunoprecipitation and Western blotting for HA and Pin1. The asterisk denotes CPEB HA 48–183.