Fig 5.
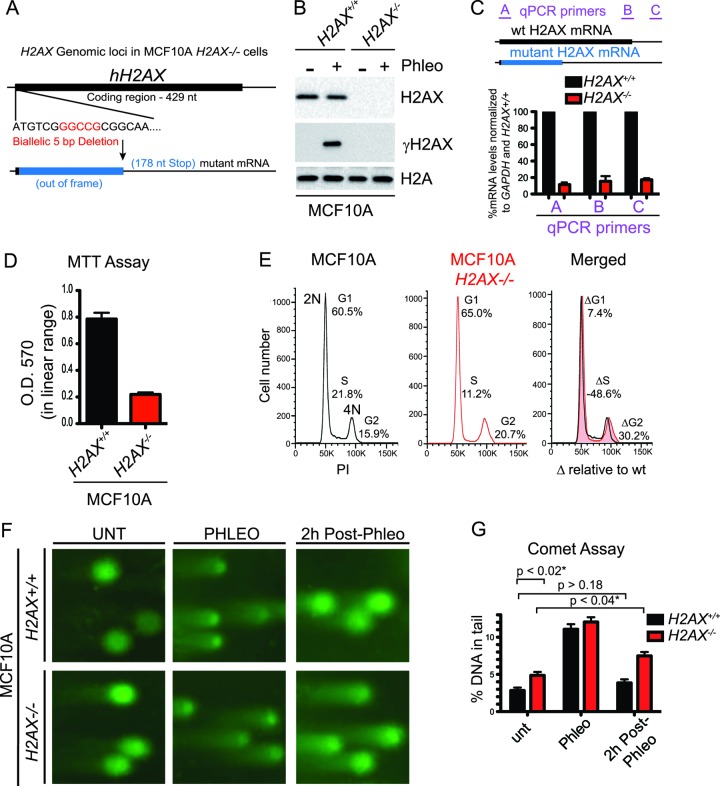
Deletion of H2AX in human cells results in cell cycle and growth defects. (A) Schematic of H2AX genomic loci in human MCF10A H2AX−/− cells. ZFN-mediated knockout of H2AX resulted in a biallelic 5-bp deletion resulting in a truncated and out-of-frame mRNA transcript. (B) Human MCF10A H2AX−/− cells lack H2AX. Loss of H2AX was confirmed by Western blotting with H2AX and γH2AX antibodies in wild-type and H2AX knockout MCF10A cells. H2A is a loading control. Cells were damaged as in Fig. 3B. (C) mRNA from the H2AX locus is reduced in MCF10A H2AX−/− cells. RNA was isolated from both MCF10A H2AX+/+ and H2AX−/− cells and analyzed by RT-qPCR with primers specific for the H2AX locus and GAPDH. The locations of H2AX locus-specific qPCR primers A, B, and C are indicated. Data were normalized to GAPDH and MCF10A H2AX+/+ samples. The graph represents data from 3 independent experiments, and error bars represent SEM. (D) Reduced proliferation in MCF10A H2AX−/− cells. MTT assays were performed on the indicated cell lines as described in Materials and Methods. O.D., optical density. Error bars represent SEM. (E) Cell cycle analysis by flow cytometry (FACS). Wild-type and H2AX-deleted MCF10A cells were analyzed by FACS with propidium iodide (PI) staining. DNA contents (2N and 4N) are indicated, and the DNA profiles are compared (overlaid right panel). The Δ cell cycle values represent percentages of the indicated cell cycle phase in MCF10A H2AX−/− cells relative to MCF10A cells. (F) MCF10A H2AX−/− cells accumulate DNA double-strand breaks (DSBs). Wild-type and MCF10A H2AX−/− cells were either untreated (UNT) or were treated with 60 μg/ml of phleomycin for 2 h followed by 2 h of recovery. Samples were analyzed by a neutral comet assay to detect DSBs. (G) Quantification of experiments from panel F. Data were quantified in Cometscore and are represented as the percentage of DNA in the tail. Error bars represent SEM of five independent experiments. P values are indicated for some comparisons. *, statistically significant at P < 0.05.