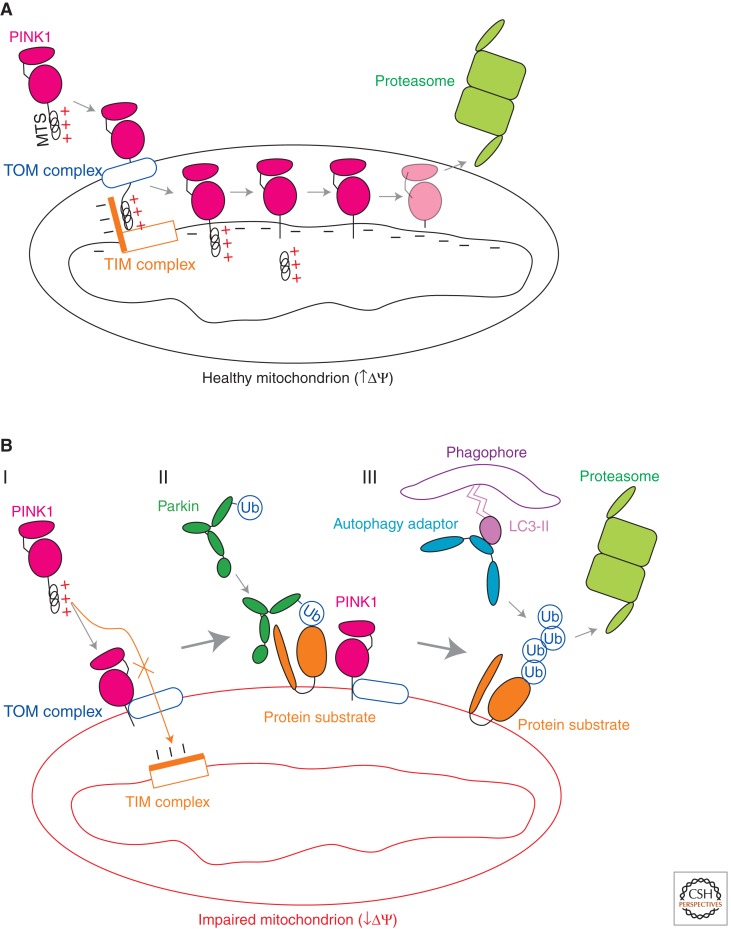
Figure 1.
Regulation of the PINK1-Parkin mitochondrial quality control pathway by inner mitochondrial membrane potential. (A) In healthy mitochondria, PINK1 is imported to the inner mitochondrial membrane by the TOM (translocase of the outer membrane) and TIM23 (translocase of the inner membrane 23) complexes, along a path that depends on the voltage component of the mitochondrial inner membrane potential (ΔΨ). At the inner membrane, the mitochondrial-targeting signal (MTS) of PINK1 is cleaved by the matrix metalloprotease. PINK1 is cleaved again in its transmembrane domain by the rhomboid protease PARL. The resulting PINK1 cleavage product is unstable and is degraded in a manner that depends on proteasomal activity. (B, I) In impaired mitochondria with collapsed ΔΨ, PINK1 cannot be imported to the inner membrane along the ΔΨ-dependent TIM23 pathway. Instead, PINK1 is directed to the outer mitochondrial membrane by a cryptic signal amino terminal to its transmembrane domain, in which it associates with the TOM complex. (B, II) The accumulation of PINK1 on the outer mitochondrial membrane recruits Parkin from the cytosol and activates its ubiquitin ligase activity. Parkin ubiquitinates outer membrane proteins preferentially on the mitochondrion on which PINK1 has accumulated. (B, III) Ubiquitination of outer mitochondrial membrane proteins by Parkin leads either to their degradation by the proteasome or to the recruitment of ubiquitin-binding adaptor proteins to effect the removal of the damaged mitochondrion by autophagy.