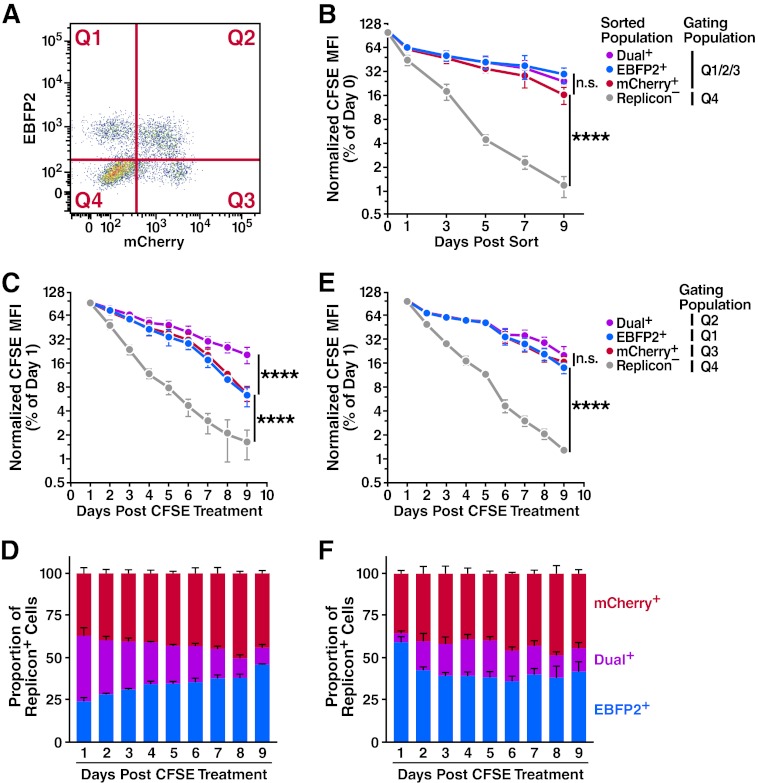
Fig 4.
Progression through the cell cycle is important in the dual- to single-replicon decay process. Huh7.5 cells were transfected with Jc1/ΔEBFP2 and Jc1/ΔmCherry RNA and treated with 5 or 10 μM CFSE 24 h later. (A) Representative flow cytometry plot demonstrating gating quadrants. (B) No difference in proliferation of isolated dual- and single-replicon cells assessed by CFSE dilution. Two days after transfection, dual-replicon, single-replicon, and replicon-negative cells were isolated by FACS. Cells were analyzed for CFSE/EBFP2/mCherry by flow cytometry; the gating quadrants represent the cells used in the analysis. Values were normalized to the CFSE mean fluorescence intensity (MFI) at day 0 postsorting (n = 4). (C) In mixed-replicon cultures, dual-replicon cells dilute CFSE more slowly than single-replicon cells. Cells were not isolated by cell sorting; instead, each population was separated by the given quadrant gates (n = 3). (D) Decay of dual-replicon cells in mixed cultures. The chart indicates the proportion of single- and dual-replicon cells in the samples in panel C. (E) No significant difference in CFSE dilution of dual- or single-replicon cells in mixed-replicon DMSO-treated cultures. Huh7.5 cells were treated for 15 days with 1% DMSO to induce a differentiated, growth-arrested phenotype before transfection and were otherwise treated as in panel C (n = 3). (F) DMSO treatment of cells slows the dual-replicon decay process. The chart indicates the proportion of single- and dual-replicon cells in the samples in panel E. Error bars indicate SEM (****, P < 0.001; n.s., nonsignificant by extra sum of squares F test using semi-log regression).