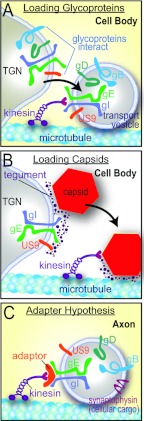
Fig 1.
Models for how HSV gE/gI and US9 might promote anterograde axonal transport of capsids and glycoproteins. (A) The loading hypothesis suggests that gE/gI and US9 accumulate in the TGN of neuron cell bodies and cause other HSV membrane proteins to accumulate there. Transport vesicles that bud from these specific TGN membranes are loaded onto kinesins for transport into proximal axons. Similar vesicles containing enveloped HSV (Married) particles might also be loaded in this way. (B) The loading of capsids onto kinesin motors may similarly be affected by gE/gI and US9 accumulation in the TGN. gE/gI is known to extensively interact with tegument proteins that, in turn, interact with capsids. Thus, by causing the accumulation of capsids in TGN loading compartments, gE/gI and US9 may promote the axonal transport of unenveloped capsids. (C) The adaptor hypothesis functions more extensively in axons rather than cell bodies. In this model, the cytoplasmic domains of gE/gI and US9 interact with kinesin adaptors or directly with kinesins to tether vesicles containing other HSV glycoproteins (gD and gB) and cellular cargo (synaptophysin) onto motors during transport.