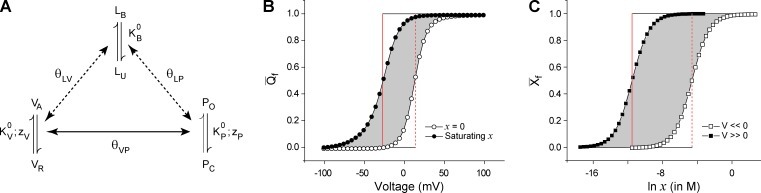
Figure 3.
Energetic linkage between the voltage-dependent and ligand-dependent pathways. (A) An allosteric model of a hypothetical ion channel with a single voltage-sensing and ligand binding domain. The voltage sensor exists in two states (VR and VA) and its activation energetics are governed by its intrinsic equilibrium constant, , and its voltage dependence, zV. The pore unit has an intrinsic activation constant and intrinsic voltage dependence given by and zP, respectively. The ligand binding domain has an intrinsic binding affinity of . All the three “structural units” are allosterically coupled to each other via interactions represented by the terms θVP, θLV, and θLP. The model parameters are = 0.1, = 0.01, = 100 M−1, θVP = 100, θLV = 1, θLP = 1,000, zV = 2.5, and zP = 1.5. (B) Simulated fractional gating charge displacement (Qf vs. V) curves for this system at saturating (closed circles) and zero (open circles) ligand concentrations. The red vertical line is the VM axis for each of the curves and the shaded area is equal to the area of the rectangle bound the two VM axes. This area, when scaled by Qmax of the system (zP + zV), gives the net energetic facilitation of the voltage-dependent pathway by the ligand and is equal to −kBTln(θLVθLP). (C) The fractional ligand binding curves (Xf vs. ln x) at very low (open circles) and very high voltages (closed circles) are shown, and the red vertical lines are the corresponding ln xm axes. The area of the shaded region is equal to area of the rectangle bound the two ln xm axes, which when scaled by Nmax of the system (equal to 1 in this case) and kBT, gives the net energetic facilitation of the ligand-dependent pathway by voltage and is equal to −kBTln(θLVθLP). Note that the two shaded in areas in B and C after appropriate scaling will be equal.