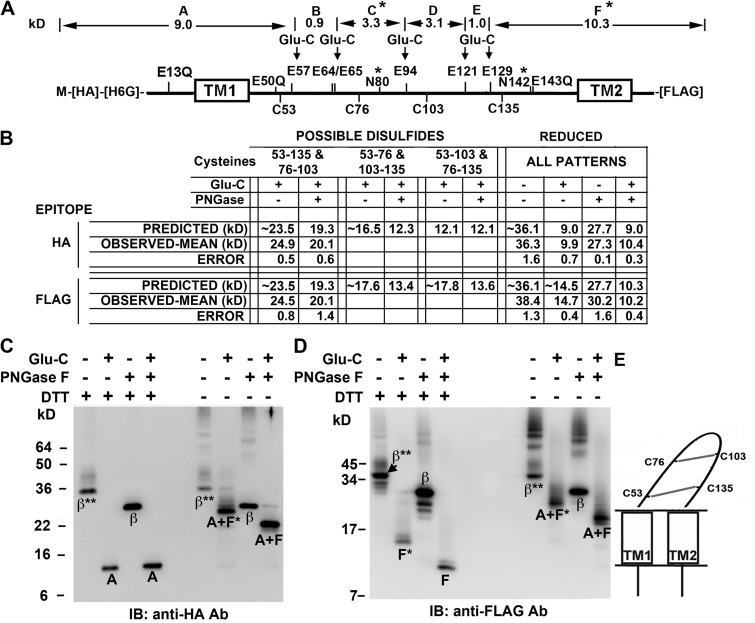
Figure 3.
Determination of disulfide cross-linking pattern of Cys in the β1 extracellular loop. (A) Locations of Cys, Glu, Glu mutated to Gln, and the two N-glycosylation sites (*) in HA-His6Gly-β1-FLAG are labeled above and below the thick line representing the sequence. Above the sequence are the molecular weights of the fragments between the remaining Glu, labeled A–F; these would result from complete cleavage by GluC endoproteinase of the fully reduced and deglycosylated subunit. (B) The predicted and observed molecular weights for the GluC proteolytic fragments detectable either with antibody against the N-terminal HA epitope or with antibody against the C-terminal FLAG epitope, for the three possible combinations of disulfides. These N- and C-terminal fragments are arranged by whether or not they are deglycosylated. To the right are the molecular weights of the N- and C-terminal GluC fragments of the fully reduced subunit. Predicted molecular weights of N-and C-terminal fragments were calculated based on the molecular weights of the fragments in A; each of two glycosylation sites was presumed to add ∼4.2 kD. The observed molecular weights are the mean of at least two independent experiments and are entered below the most closely matching predicted molecular weights. (C) Immunoblot (IB) with anti-HA antibody showing subunit fragments containing the N terminus of the subunit. The treatments of the samples match those in the table in B. *, single glycosylation site; **, two glycosylation sites. (D) Immunoblot with anti-FLAG antibody showing subunit fragments containing the C terminus. (E) Schematic of β1 depicting the only disulfide cross-linking pattern consistent with the observed fragment molecular weights.