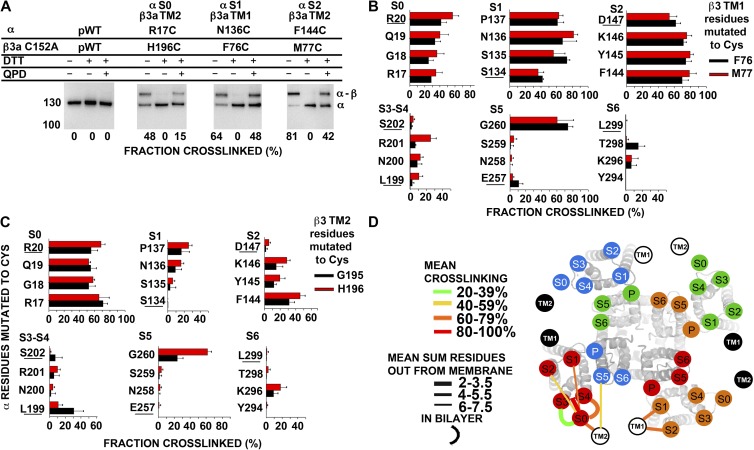
Figure 7.
Position of extracellular flanks of mβ3a relative to the α S1–S6 TM helices. (A) Immunoblots illustrating endogenous cross-linking of Cys substituted in the extracellular flanks of α and C152A mβ3a. Expression of mutants, selection of channels transported to the cell surface, SDS gel electrophoresis, and blotting are described in Materials and methods. The blots were developed with an antibody against an epitope from the C terminus of α, which recognizes α (∼130 kD) and the cross-linked α–mβ3a complex (∼160 kD). Portions of each sample were reduced with DTT and oxidized for 20 min with 40 µM QPD. At the bottom of each lane is the fraction of the cross-linked α–β3 complex. (B and C) Extents of endogenous disulfide bond formation between Cys-substituted extracellular flanks of α S0–S6 with mβ3a TM1 (B) and mβ3a TM2 (C) flanks. The α residues substituted by Cys are color coded as shown. (D) Locations of the extracellular ends of mβ3a TM1 and TM2 relative to the extracellular ends of α S0–S6. The locations of the extracellular ends of mβ3a TM1 and TM2 are represented by labeled black and white circles. TM1 and TM2 from two mβ3a subunits are represented by white circles with black letters, and two mβ3a subunits are represented by black circles with white letters. For each pair of flanks, the mean of the top three extents of cross-linking (see Materials and methods) is coded by the color of the connecting line (see legend in panel). To indicate the distance of a cross-link from the ends of the TM helices, we added the number of residues out from the membrane of each of the two Cys. The mean of these distances for the top three extents of cross-linking is indicated by the thickness of the line (see legend in panel). The position of S0 relative to S1–S6 was determined as described in Liu et al. (2008a, 2010). P, pore.