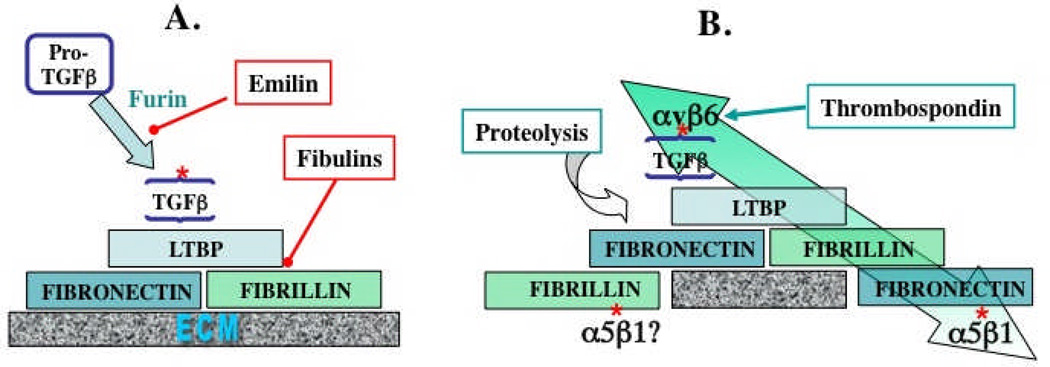
Figure 2. ECM interactions regulating TGFβ.
A. Incorporation into the ECM.
Cleavage by furin protease of Pro-TGFβ to the small latent complex (SLC) comprising TGFβ and LAP is inhibited by emilin, an ECM protein. The SLC binds to LTBP, via S-S bonding to a TB domain, to form the large latent complex (LLC), in which form the TGFβ is inactive21,22. LTBP then binds to fibrillin and to fibronectin (see Figure 1 for specific interaction domains). Fibulins compete for LTBP binding to fibrillin39. Fibrillin binds to preexisting fibronectin fibrils or assembles into microfibrils and both fibrillin and fibronectin undergo further homomeric and heteromeric interactions within the ECM.
B. Activation of ECM-bound latent TGFβ.
TGFβ can be activated by proteolysis of the ECM proteins and/or of LAP or directly by thrombospondin (see text). TGFβ can also be activated by mechanical strain (large green arrow). This strain arises from cytoskeletal force applied through αvβ6 integrin, which binds to an RGD site in LAP and requires attachment of the TGFβ/LAP complex through LTBP to the fibronectin-rich matrix, which is, in turn, attached via α5β1 integrin to other cells. Fibrillin might also be attached to cells via integrins.