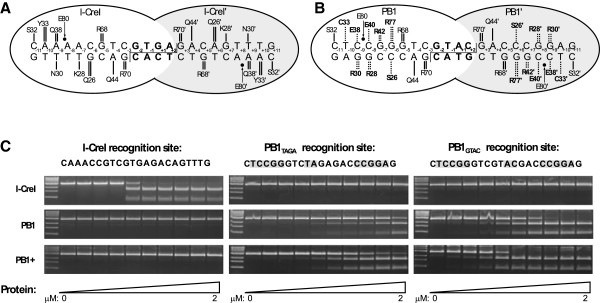
Figure 1.
DNA-protein interactions of endonucleases and their in vitro cleavage of distinct DNA substrates. (A) Diagram of wild-type I-CreI homodimer in complex with its natural DNA recognition sequence. One I-CreI monomer is shown in white, the other (I-CreI’) in grey. DNA sequence is indicated, with four base-pair center sequence shown in bold. Direct hydrogen bonds between I-CreI and DNA are shown as black lines. Sites of phosphodiester bond cleavage and the resulting 4 bp 3′ overhangs are indicated by a line. A likely unfavorable electrostatic interaction between E80 and a backbone phosphate is indicated by a small arrow. (B) Predicted interactions between rationally designed PB1 endonuclease and the RSGTAC DNA site. The two monomers (PB1 and PB1′) and DNA interactions are as indicated in (A), except amino acids that deviate from I-CreI and I-CreI’ hydrogen bonds (or a hydrophobic interaction, C33) and are predicted to contribute to altered DNA-cleavage specificity are indicated with dashed lines. PB1+ endonuclease contains a mutation (E80 to Q80) predicted to eliminate the unfavorable interaction mentioned in (A). (C) Cleavage of DNA by native and rationally designed endonucleases. I-CreI, PBI, and PB1+ endonucleases were incubated with three distinct linearized DNA substrates (sequence indicated above its respective set of digests). Sequence differences between I-CreI (wild-type) and the two PB1 recognition sites highlighted in grey. DNA for PB1TAGA (center) and PB1GTAC (right) differ from each other by the 4 bp center sequence (subscript). Digests were conducted with 0, 0.007, 0.015, 0.031, 0.062, 0.125, 0.25, 0.5, 1, 2 μM endonuclease.