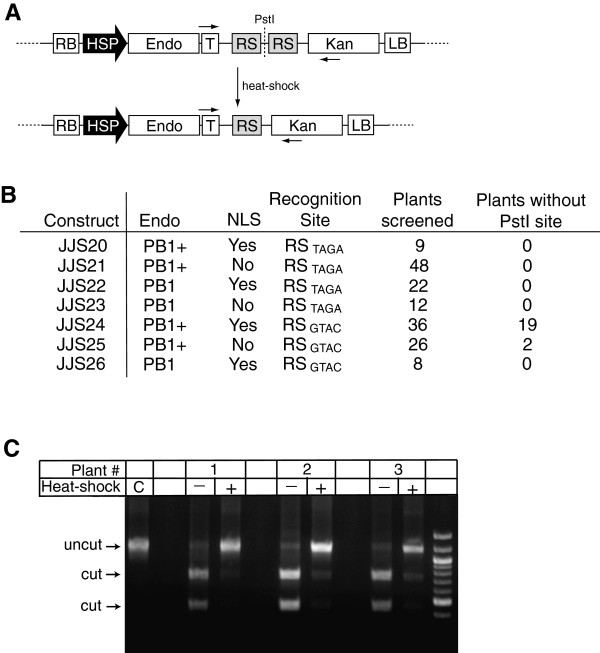
Figure 2.
In planta cleavage of PB1 recognition sites by engineered endonucleases. (A) T-DNA structure before and after induction of the endonuclease. Endonuclease cleavage excises the central fragment 5′-TTCTGCAG-3′, eliminating the indicated PstI site. RB, right border; HSP, Hsp18.2 promoter; Endo, PB1 or PB1+, endonuclease; T, Nopaline Synthase terminator; RS, endonuclease recognition site (RSTAGA or RSGTAC); Kan, kanamycin resistance marker; LB, left border. Horizontal arrows indicate approximate locations of PCR primers used for diagnostic evidence of in planta endonuclease cleavage. (B) Table of experimental results. Seven different T-DNA constructs used in this study, with the general form diagramed in (A). Each T-DNA has three possible differences: presence (Yes) or absence (No) of a nuclear localization signal (NLS) on the endonuclease; the endonuclease with either the lower activity PB1 or higher activity PB1+ (containing Q80E mutation) PB1 recognition sites (RS) contain either a TAGA or GTAC central 4 bp sequence. Plants containing some constructs (JJS20, 23, and 26) had a low recovery rate after heat shock treatment, resulting in a lower number of plants screened. (C) Sample agarose gel data showing loss of the PstI restriction site from genomic DNA following heat-shock treatment of JJS24 plants. The agarose gel shows three JJS24 samples that demonstrated loss of the PstI site. Control (C) shows size of uncut PCR fragment. PCR fragments from samples before heat shock (–) are cut >90% into smaller bands (identified as “cut” on left). After heat shock (+), PCR fragments from the three samples are largely uncut by PstI, indicating a loss of the PstI site in planta.