Abstract
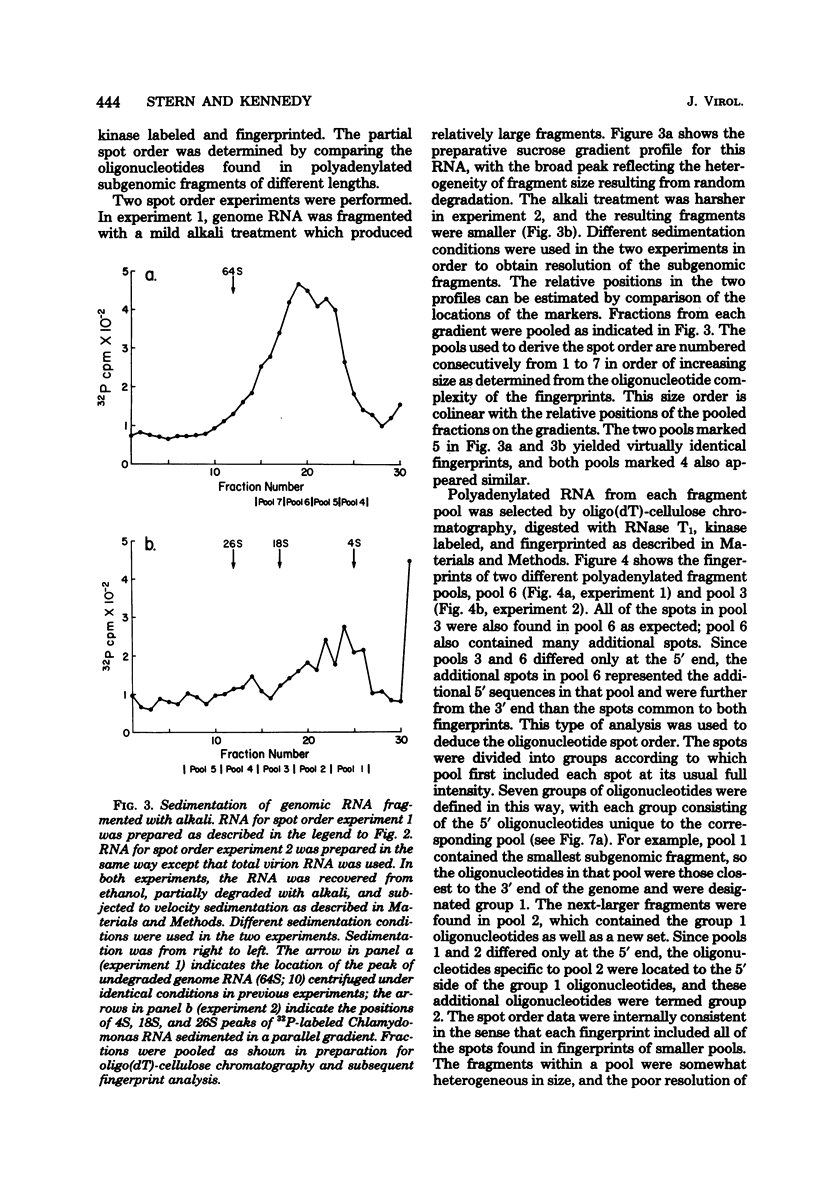
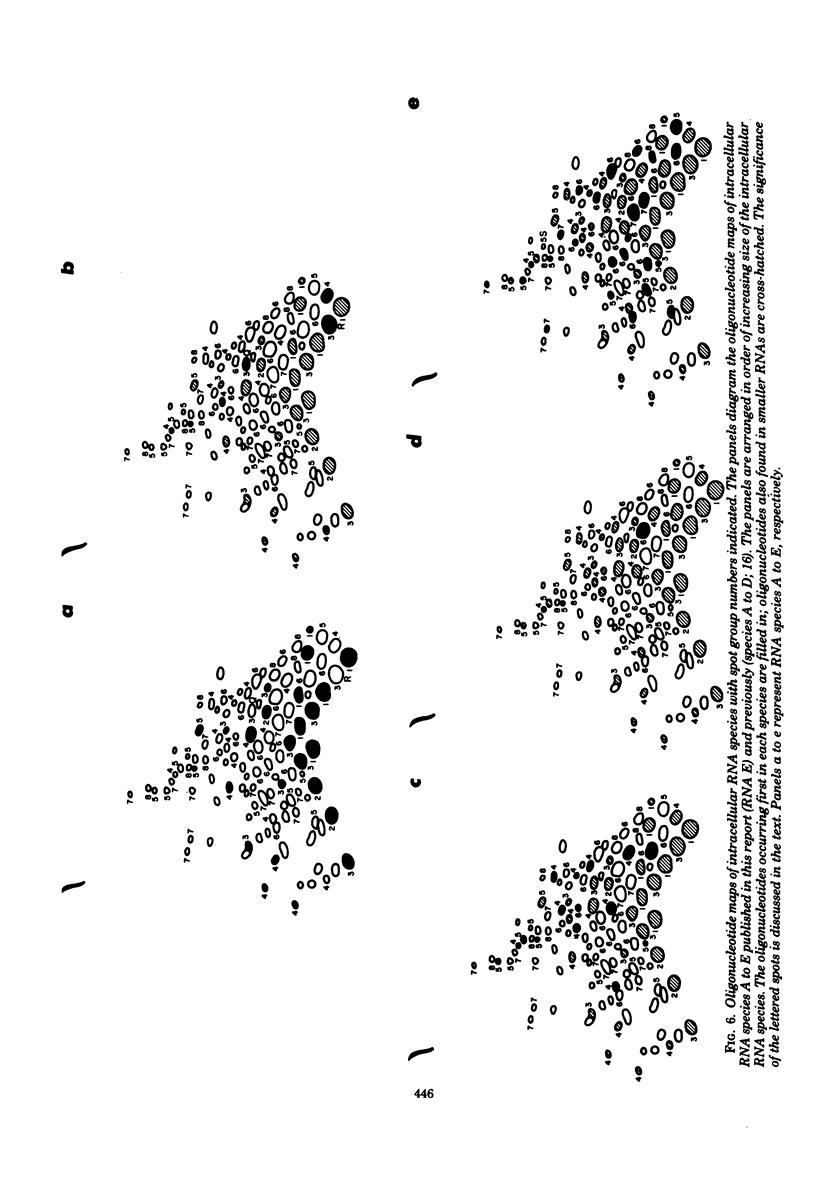
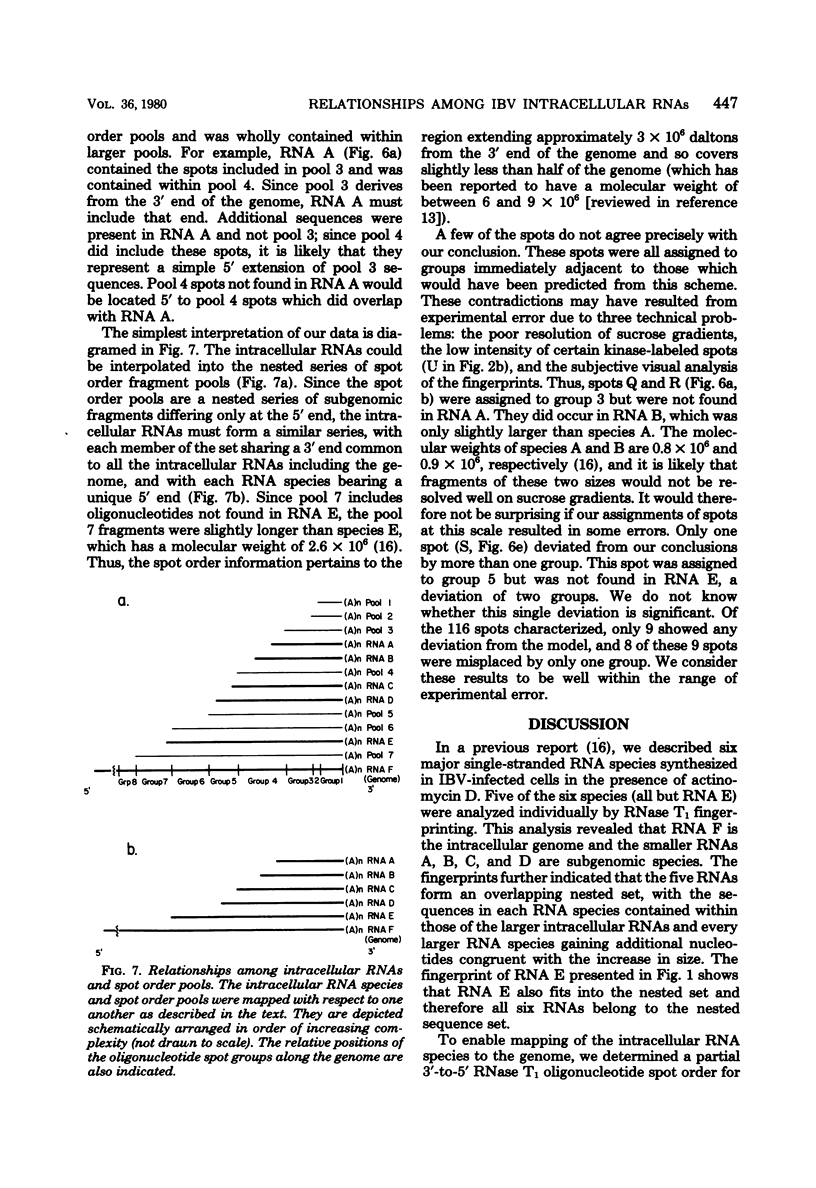
Avian infectious bronchitis virus, a coronavirus, directed the synthesis of six major single-stranded polyadenylated RNA species in infected chicken embryo kidney cells. These RNAs include the intracellular form of the genome (RNA F) and five smaller RNA species (RNAs A, B, C, D, and E). Species A, B, C, and D are subgenomic RNAs and together with the genome form a nested sequence set, with the sequences of each RNA contained within every larger RNA species (D. F. Stern and S. I. T. Kennedy, J. Virol 34:665-674, 1980). In the present paper we show by RNase T1 oligonucleotide fingerprinting that RNA E is also a member of the nested set. Partial alkaline fragmentation of the genome followed by sucrose fractionation, oligodeoxythymidylate-cellulose chromatography, and RNase T1 fingerprinting gave a partial 3'-to-5' oligonucleotide spot order. A comparison of the oligonucleotides of each of the five subgenomic RNAs with this spot order established that all of the RNAs are comprised of nucleotide sequences inward from the 3' end of the genome. This result is discussed in relation to the multiplication strategy both of coronaviruses and of other RNA-containing viruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black D. N., Burroughs J. N., Harris T. J., Brown F. The structure and replication of calicivirus RNA. Nature. 1978 Aug 10;274(5671):614–615. doi: 10.1038/274614a0. [DOI] [PubMed] [Google Scholar]
- Brzeski H., Kennedy S. I. Synthesis of Sindbis virus nonstructural polypeptides in chicken embryo fibroblasts. J Virol. 1977 May;22(2):420–429. doi: 10.1128/jvi.22.2.420-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzeski H., Kennedy S. I. Synthesis of alphavirus-specified RNA. J Virol. 1978 Feb;25(2):630–640. doi: 10.1128/jvi.25.2.630-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Polyadenylic acid sequences in the virus RNA species of cells infected with Semliki Forest Virus. J Gen Virol. 1974 Mar;22(3):331–345. doi: 10.1099/0022-1317-22-3-331. [DOI] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W., Chanock R. M., Lai C. J. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1857–1861. doi: 10.1073/pnas.77.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Kennedy I. Genome of infectious bronchitis virus. J Virol. 1977 Oct;24(1):99–107. doi: 10.1128/jvi.24.1.99-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The characterisation of the virion RNA of avian infectious bronchitis virus. FEBS Lett. 1977 May 15;77(2):311–313. doi: 10.1016/0014-5793(77)80258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Kennedy S. I. Semliki forest virus persistence in mouse L929 cells. Virology. 1980 Jan 15;100(1):141–155. doi: 10.1016/0042-6822(80)90560-7. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Stevens R. H., Simpson R. W. Presence of infectious polyadenylated RNA in coronavirus avian bronchitis virus. Virology. 1977 Apr;77(2):772–782. doi: 10.1016/0042-6822(77)90498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Watkins H., Reeve P., Alexander D. J. The ribonucleic acid of infectious bronchitis virus. Arch Virol. 1975;47(3):279–286. doi: 10.1007/BF01317815. [DOI] [PMC free article] [PubMed] [Google Scholar]






