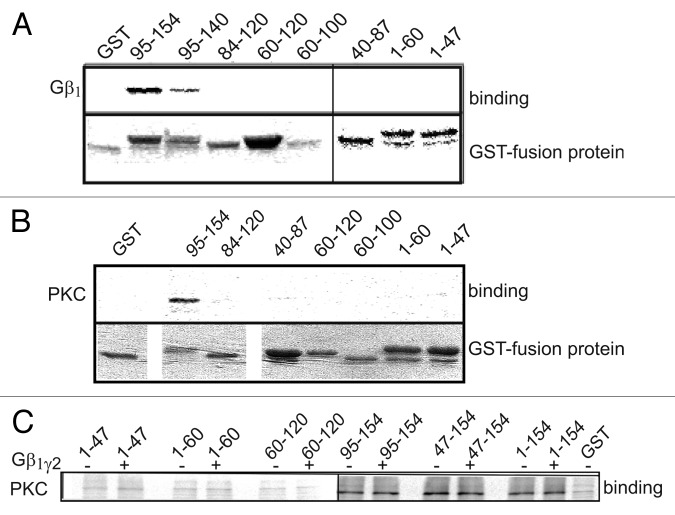
Figure 5. Both Gβγ and PKCα bind distal segments of α1C-NT. (A) Binding of [35S]-labeled Gβ1γ2 to different GST-fused fragments of the NT. Immobilized GST fusion proteins were incubated with Gβ1γ2. After washing, Gβ1γ2-bound proteins were eluted and resolved by SDS-PAGE. Gβ1γ2 was found to bind the distal third of the NT. Similar data was obtained in six more experiments. (B) Binding of [35S]-labeled PKCα to different GST-fused fragments of the NT. PKCα was found to bind the distal half of the NT. (C) [35S]PKCα binding to segments of the NT was repeated in the presence of 1.5 μg purified Gβ1γ2 . It did not alter PKC binding to the NT, suggesting no binding competition for the same site.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.