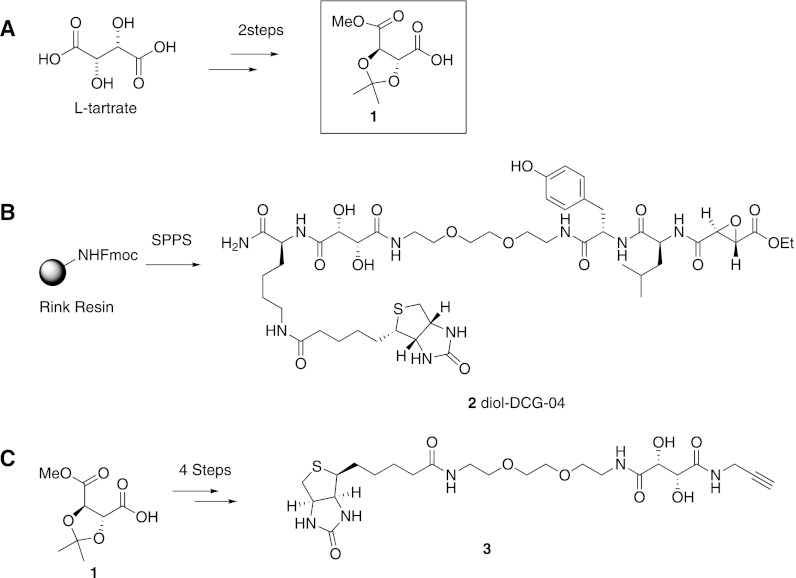
Fig. 2.
Synthesis of the cleavable linker building block and its probe derivatives. A, the synthesis of cleavable building block 1 from l-tartaric acid can be achieved in two easy protecting group manipulation steps: protection of the functional groups with an isopropylidene and methyl esters, followed by selective saponification of one methyl ester. B, diol-DCG-04 (2) was made via Fmoc-based solid phase peptide synthesis. C, cleavable alkyne biotin reagent 3, which is amenable for click chemistry mediated labeling of azido-tagged proteins, can be made in four steps from building block 1.