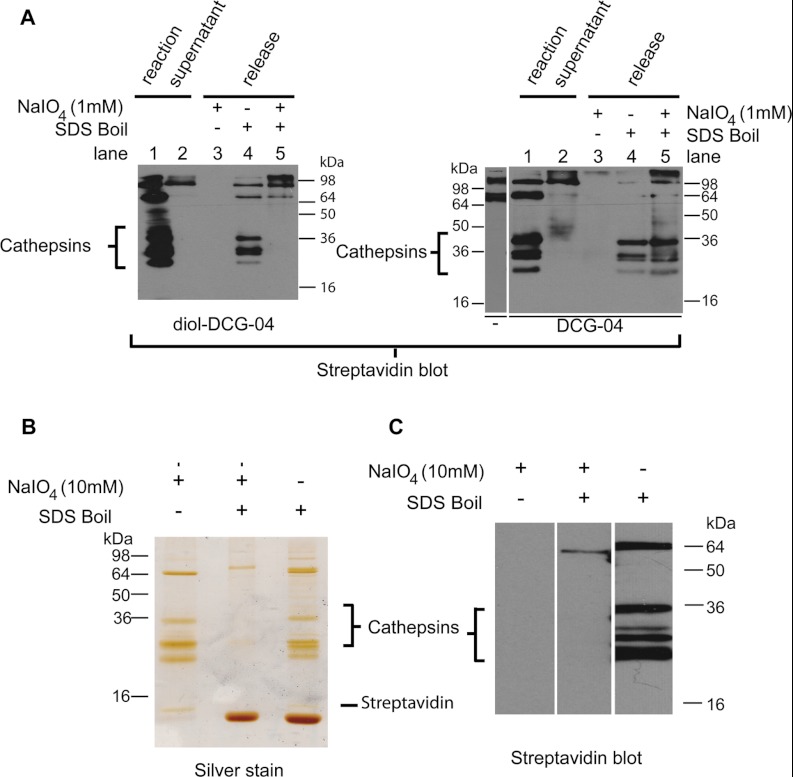
Fig. 3.
Elution of enriched rat liver lysate cathepsins. A, cathepsins labeled by diol-DCG-04 (left gel) or DCG-04 (right gel) can be efficiently pulled down by streptavidin beads (compare lanes 1 and 2). They are released by boiling (lane 4). Treatment with NaIO4 (lane 3) does not show any cathepsin bands, because they have lost their biotin (diol-DCG-04; left gel) or are not eluted (DCG-04; right gel). Subsequent boiling of these samples releases the remaining biotinylated proteins. For DCG-04, both endogenously biotinylated proteins and probe-labeled cathepsins are eluted. For diol-DCG-04, only endogenously biotinylated proteins are eluted, with none of the cathepsins, indicating efficient release by NaIO4. B, selective (10 mm NaIO4) or non-selective (SDS sample buffer boil) release of cathepsins from streptavidin beads detected via silver staining. Boiling of the beads after selective release does not elute additional cathepsin proteins. C, streptavidin Western blot of the same samples as in the silver staining. Note that the NaIO4 cleaved proteins are invisible because of the chemoselective removal of the biotin part.