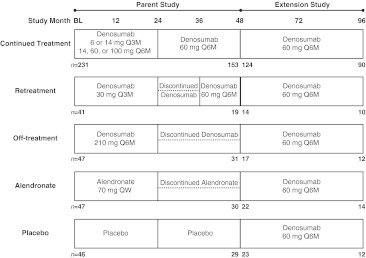
Fig. 1.
Study design of the 4-year parent dose-ranging study with the different treatment regimens at months 24 and 48, and the 4-year extension study with all subjects receiving open-label denosumab 60 mg every 6 months. n = number of subjects who enrolled in the parent and extension study and those that completed each study