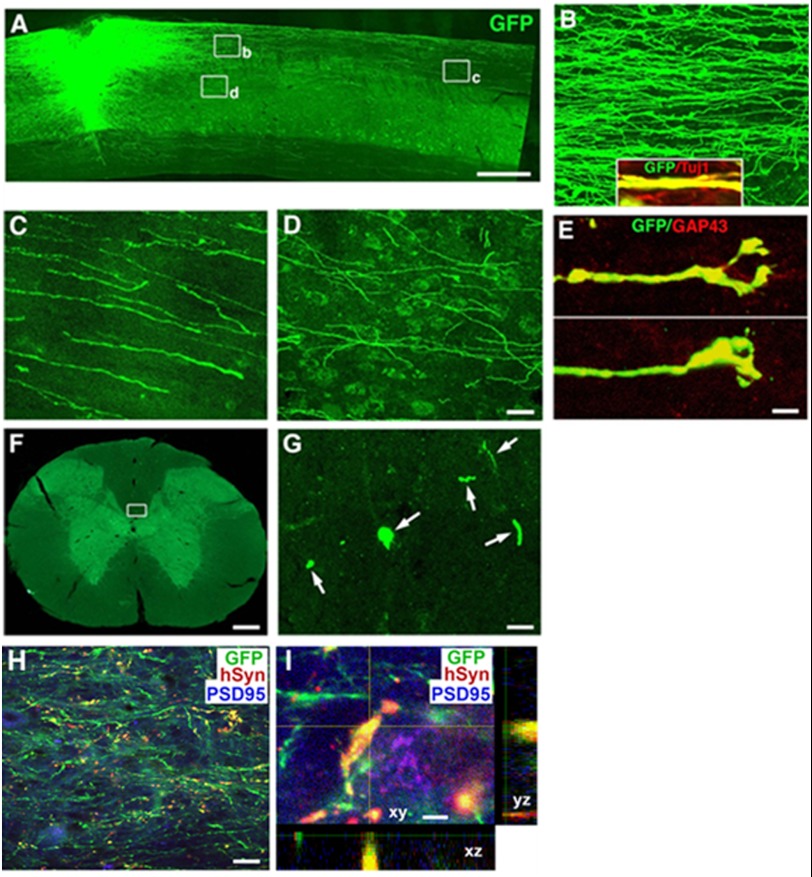
FIGURE 4.
Long distance axonal growth and integration of transplanted pNSCs. GFP-expressing pNSCs robustly extended axons into the host spinal cord caudal and rostral to the C3 dorsal column lesion site (rostral is shown) (A). Higher magnification views show that axons traveled mainly in host white matter (B and C) and that some axons innervated the gray matter (D). The inset in B shows that GFP-labeled projections arising from the graft expressed Tuj1, confirming an axonal identity. The tips of the growing axons featured growth cone-like structures (E) that co-localized with GAP43. Individual GFP-expressing axons can be detected at the C1 (shown) or C7 (F and G) spinal cord level, three segments rostral or caudal to the spinal cord lesion/graft site. G is a higher magnification of the boxed area in F showing individual axons in the dorsal column (arrows). GFP, human-specific synaptophysin (hSyn), and PSD-95 labeling revealed that numerous pNSC-derived axon terminals (green) in the host gray matter expressed synaptophysin (1 mm caudal to the lesion site) surrounding PSD-95-expressing host neurons (H). A high magnification z-stack image (I) triple-labeled for GFP, human-specific synaptophysin, and PSD-95 demonstrates co-localization of graft-derived axons with the human-specific synaptic marker in direct contact with host neurons expressing the post-synaptic marker PSD-95, indicating sites of putative synaptic connections. Scale bars = 650 μm (A), 32 μm (B–D), 5 μm (E), 300 μm (F), 8 μm (G), 30 μm (H), and 3 μm (I).