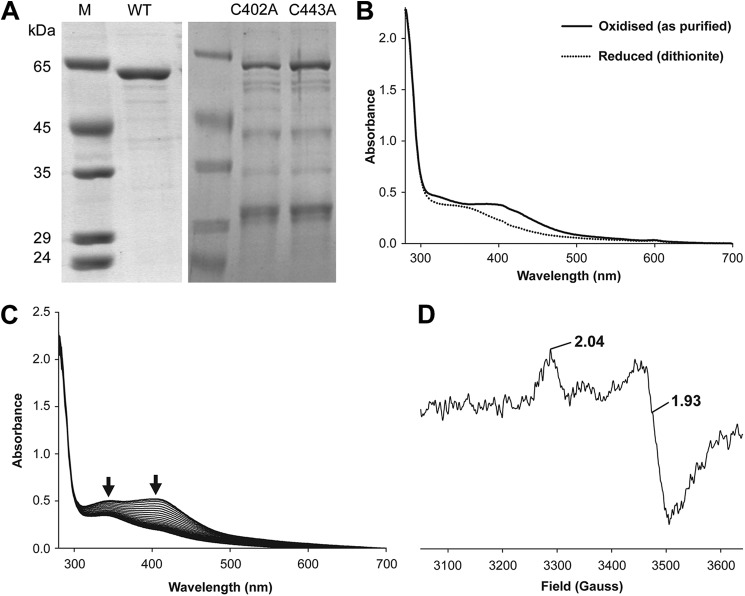
FIGURE 2.
Purified B. megaterium CbiH60 UV-visible and EPR spectra. A, SDS gel showing purified CbiH60 proteins used in this study. The molecular mass markers are shown under lane M; lane WT contains ∼3 μg of CbiH60; lane C402A contains ∼1.5 μg of C402A CbiH60; and lane C443A contains ∼1.5 μg of C402A CbiH60. B, UV-visible spectra of 50 μm CbiH60 as purified (solid line) and after reduction with excess dithionite (dotted line). C, UV-visible spectra of 50 μm CbiH60 exposed to air and scanned every 10 min. Arrows show a loss of absorbance between 300 and 450 nm, indicating the loss of the [4Fe-4S] cluster. D, EPR of 200 μm (12 mg ml−1) CbiH60 (buffer-exchanged in Buffer H), reduced with excess dithionite (10 mm) and frozen in liquid nitrogen. From the intensity of the signal, ∼20–25 μm (∼10%) of reduced [4Fe-4S]1+is present. The [4Fe-4S]1+ spectrum of CbiH60 was recorded at 12 K, employing a microwave frequency of 9.383984 GHz, microwave power of 1 milliwatt, a modulation frequency of 100 kHz, and a modulation amplitude of 5 G. A spectrum of CbiH60 was also recorded at 70 K, which resulted in a loss of the [4Fe-4S]1+ signal. This indicated t1hat the iron-sulfur center was of the [4Fe-4S] class.