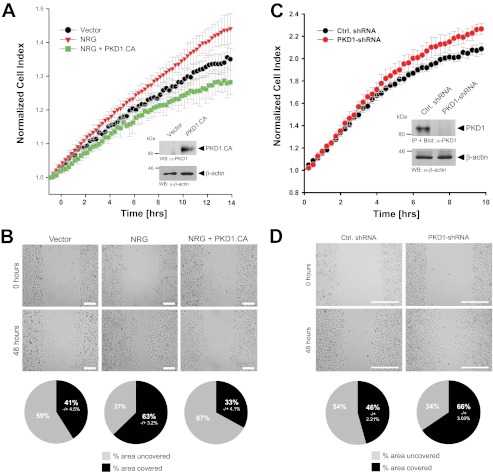
FIGURE 2.
Inhibition of PKD1 activity by NRG leads to increased cell motility. A and B, MCF-7 cells were transfected with a vector control or constitutively active PKD1 (PKD1.CA). Cells were seeded on Transwell CIM-Plate 16 plates and subjected to real-time migration assays (xCELLigence) over a time period of 14 h (A). Error bars (gray) represent three experiments. Control Western blotting (WB) was performed to determine overexpression of constitutively active PKD1. Equal loading was controlled by probing for β-actin (anti-β-actin antibody). Cells were also subjected to wound healing assays over a time period of 48 h. The area closure of the scratch wound was quantified (Image-Pro Plus), and the pie graphs show the percent area covered or uncovered (B). C and D, MCF-7 cells stably expressing shRNA directed against PKD1 or control (Ctrl.) virus were seeded in Transwell CIM-Plate 16 plates and, after adhesion, subjected to real-time migration assays (xCELLigence) over a time period of 10 h. Error bars (gray) represent three experiments. Additionally, lysates of cells were analyzed for efficient knockdown of PKD1. PKD1 was immunoprecipitated (IP; anti-PKD1 antibody), subjected to SDS-PAGE, and transferred to nitrocellulose, and samples were stained with PKD1-specific antibody. Western blot staining for β-actin served to show equal loading. Cells were also subjected to wound healing assays over a time period of 48 h. The area closure of the scratch wound was quantified (Image-Pro Plus), and the pie graphs show the percent area covered or uncovered (D).