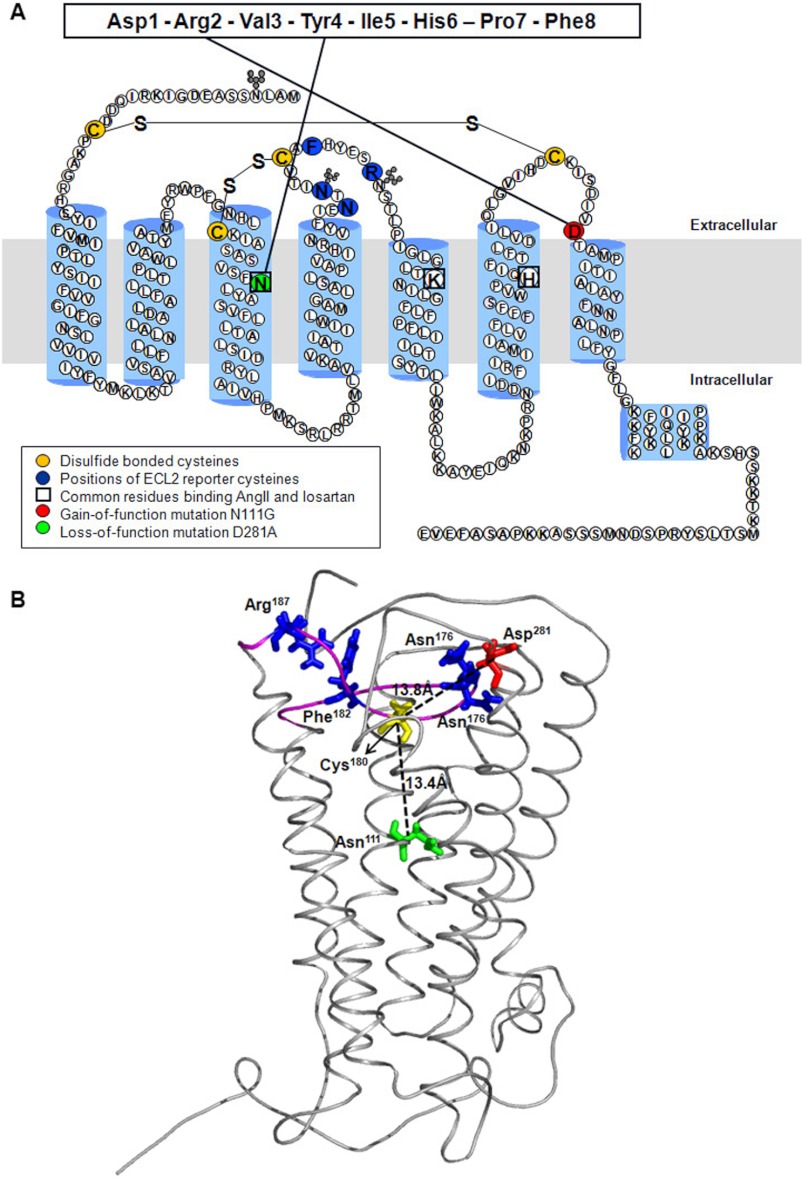
FIGURE 1.
Model of rat AT1R. A, secondary structure model of rat AT1R. ECL2 residues substituted with reporter cysteines are highlighted in dark blue. Cysteine residues involved in formation of disulfide bonds are shown in yellow. Nonessential cysteines are replaced with alanine as described previously (11). The gain of function substitution residue Asn111 on TMIII is highlighted in green. The loss of function substitution residue Asp281 on ECL3 is shown in a red circle. The interactions of Asn111 and Asp281 with AngII previously mapped by site-directed mutagenesis are shown with solid lines. The residues involved in both AngII and losartan binding are boxed. B, model of rat AT1R showing position of residues Asn111 and Asp281 relative to Cys180 located in the middle of ECL2 in the AT1R. The backbone Cα trace of AT1R model is shown in gray, and the ECL2 region is highlighted in magenta. The Cα-Cα distances of Asn111 (green) and Asp281 (red) from Cys180 (yellow) are shown as dashed lines. The distance between Cys180 and Asp281 in AngII- and losartan-bound states did not change substantially (not shown). The native residues replaced by Cys reporters are represented as blue sticks. PyMOL (version 0.99rc6) was used to visualize the protein structures and generate images.