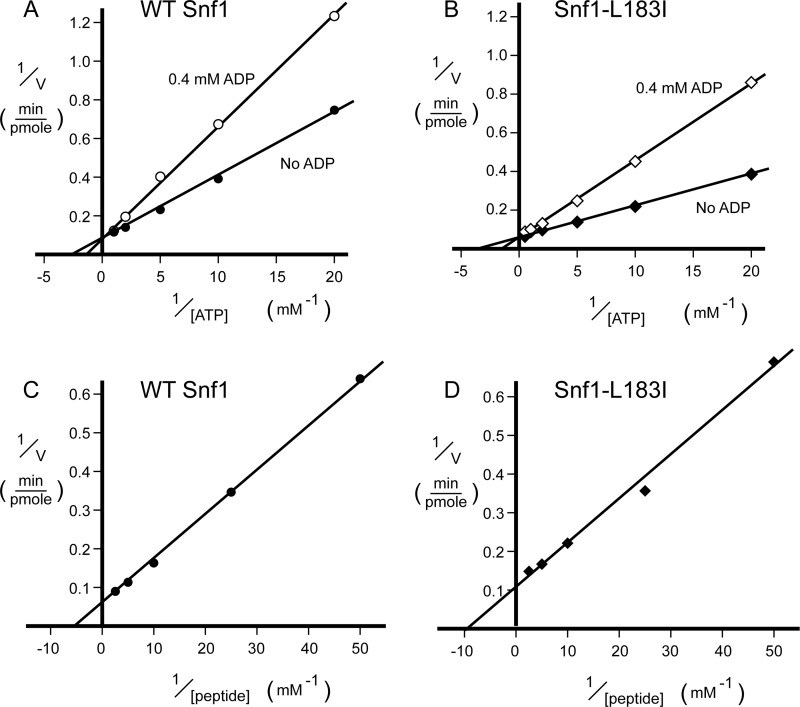
FIGURE 2.
Kinetic analysis of the wild type Snf1 and Snf1-L183I enzymes. Wild type Snf1 (A and C) and Snf1-L183I heterotrimers (B and D) containing all three subunits (Snf1, Gal83, and Snf4) were assayed for the ability to phosphorylate the SAMS peptide. Initial reaction velocities were measured and plotted as a function of substrate concentration using the double reciprocal format. Reactions with varying concentrations of ATP were conducted in the presence or absence of 0.4 mm ADP (A and B). Reactions with varying concentrations of peptide substrate were conducted in the presence of 1 mm ATP (C and D).