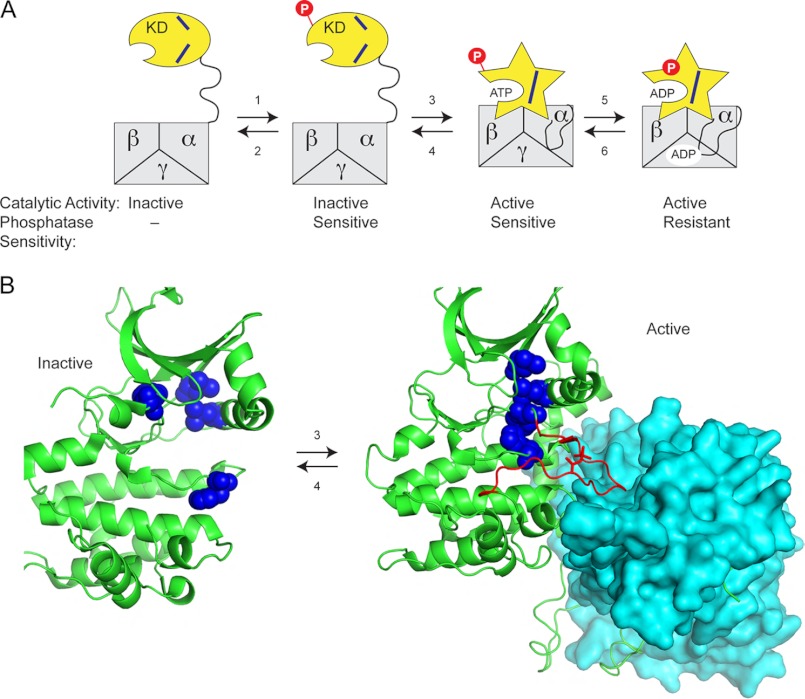
FIGURE 6.
Model for activation of Snf1 kinase and acquisition of phosphatase resistance. A, the Snf1 kinase domain (KD) is loosely tethered to the heterotrimer core (αβγ) via a disordered linker domain with the activation loop accessible to phosphorylation (reaction 1) and dephosphorylation (reaction 2). Once it is phosphorylated, the kinase domain can associate with the heterotrimer core (reaction 3) and adopt the active conformation (star shape) with the alignment of the regulatory spine (blue lines). Binding of low energy adenylate ligands to the active site and/or the γ subunit promotes formation of a phosphatase resistant conformation (reaction 5), indicated here as the phosphate (red P) burrowing into the KD. B, structure of the mammalian AMPK kinase domain (green cartoon representation) in the inactive and active states showing the position of the residues (blue spheres) that comprise the regulatory spine (residues Leu-68, Leu-79, Phe-158, and His-137 in mammalian AMPK). Upon association with the heterotrimer core (cyan surface representation), the kinase domain adopts an active conformation with the regulatory spine in alignment. The activation loop (shown in red) becomes structured when it is bound in a cleft in the heterotrimer core. Protein Data Bank files used to generate this figure were 2Y94 and 2H6D.