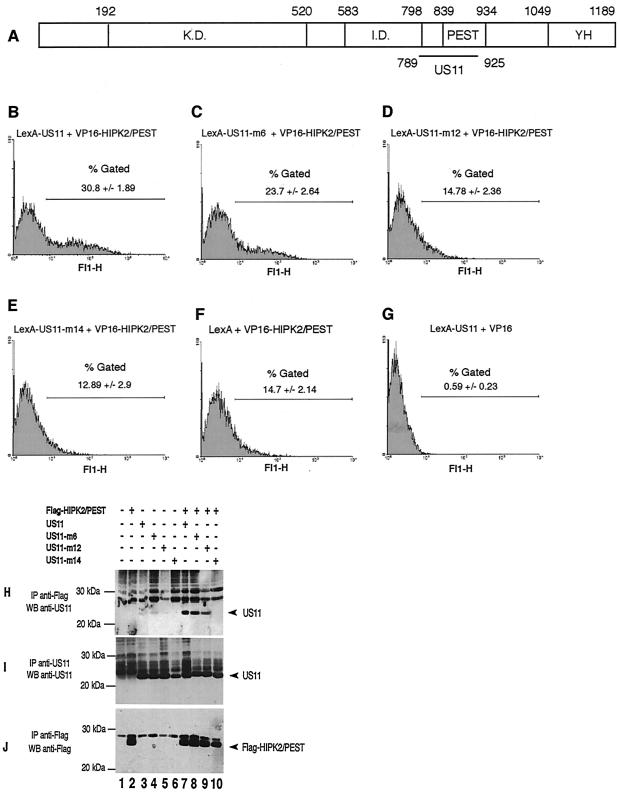
FIG. 1.
(A) Schematic representation of the HIPK2 coding sequence. K.D., kinase domain; I.D., interaction domain for homeoproteins; PEST, proline-, glutamic acid-, serine-, and threonine-rich domain; YH, tyrosine- and histidine-rich domain. Numbers indicate amino acid positions. The horizontal black line marks the delineation of the interaction domain of HIPK2 with US11. (B to G) Interaction of LexA-US11 and LexA-US11 mutant forms with VP16-HIPK2/PEST. The protein-protein interaction was measured by the yeast two-hybrid analyses with the GFP gene as a reporter. Yeast strain YRN974 with an integrated LexA operator-GFP gene was used for these analyses. (B) Yeast cells were transformed with expression vectors encoding LexA-US11 and VP16-HIPK2/PEST proteins. (C to E) Yeast cells were transformed with expression vectors encoding VP16-HIPK2/PEST combined with various vectors coding for different LexA-US11 mutants, i.e., LexA-US11-m6, LexA-US11-m12, and LexA-US11-m14. Mutations carried by these proteins are detailed in reference 5. (F) Yeast cells expressing LexA alone and VP-16-HIPK2/PEST. (G) Yeast cells expressing LexA-US11 and VP-16 alone. (H to J) Interaction of the PEST domain of HIPK2 with US11 and US11 mutants in human cells. HeLa cells were cotransfected with empty vectors (lane 1) or the indicated expression vectors containing a cDNA encoding Flag-HIPK2/PEST (lanes 2 and 7 to 10), US11 (lanes 3 and 7), US11-m6 (lanes 4 and 8), US11-m12 (lanes 5 and 9), or US11-m14 (lanes 6 and 10). Cell lysates were subjected to immunoprecipitation (IP) with an anti-Flag antibody (H and J) or an anti-US11 antibody (I). Purified proteins were analyzed by Western blotting with an anti-US11 (H and I) or an anti-Flag (J) antibody. Arrowheads indicate the positions of US11 (H and I) or Flag-HIPK2/PEST (J). Molecular masses are shown on the left in kilodaltons. +, present; −, absent.