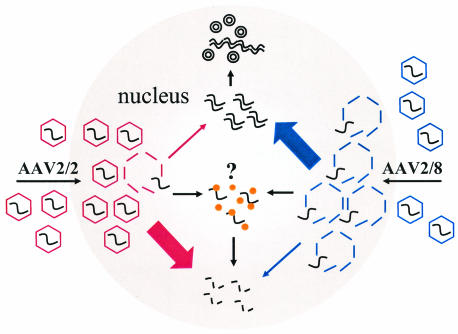
FIG. 11.
Theoretical model for rAAV transduction of the liver. AAV2 VG packaged within an AAV2 shell (AAV2/2) traffic slowly to the nucleus. Capsid disassembly is mediated by a nuclear factor(s). Upon uncoating, ss VG either become stabilized by annealing rapidly with complementary AAV genomes or they are degraded and lost. The rate of uncoating determines the proportion of VG that become stabilized by annealing or lost by degradation. The AAV2/2 pseudotype uncoats slowly. Annealing is therefore inefficient, and most of the VG are removed by degradation. A nuclear protein (such as FKBP52) might bind to the ss AAV genomes and physically obstruct annealing or target the genome for degradation. The AAV2/8 pseudotype, containing the AAV2 genome packaged within an AAV2/8 capsid, is more efficient than the AAV2/2 pseudotype because it uncoats rapidly. Annealing of complementary genomes occurs with higher efficiency, and a reduced proportion of VG are lost by degradation. Thus, AAV2/8 vectors mediate more efficient liver transduction than AAV2/2 vectors.