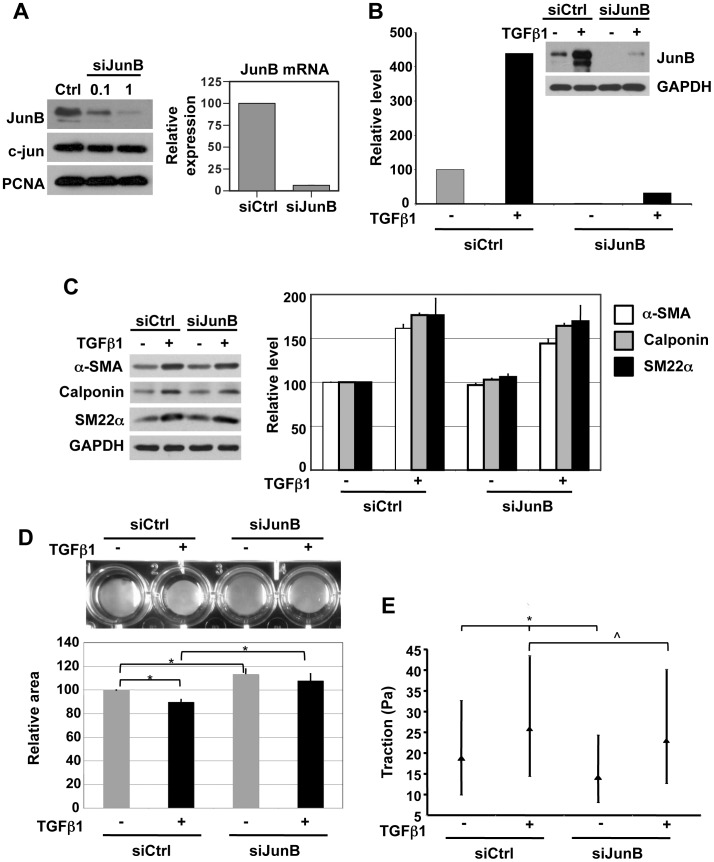
Figure 3. JunB silencing attenuates TGFβ1-induced changes in cell contractility and cytoskeletal tension, but not induction of markers of smooth muscle differentiation.
(A) BSMC were nucleofected with non-targeting control siRNA or with siRNA against JunB (0.1 µM and 1 µM) and assessed for JunB protein by immunoblotting (left panel, top). Effective knockdown of JunB was observed, with no change in c-Jun levels, demonstrating specificity of the siRNA used. Proliferating cell nuclear antigen (PCNA) expression was used as a loading control. 1 µM JunB siRNA reduced the levels of JunB mRNA by >80%, relative to non-targeting control siRNA, as assessed by semi-quantitative real-time PCR (right panel) (B) Reduction in JunB protein levels by siRNA in BSMC under basal and TGFβ1-stimulated conditions, demonstrated by immunoblotting. JunB levels were normalized to their respective GAPDH levels and expressed as percentage change relative to cells transfected with control siRNA and not subjected to TGFβ1 treatment. A representative immunoblot and its corresponding quantitation are shown. (C) TGFβ1-mediated induction of α-smooth muscle actin (α-SMA) calponin and SM22α, markers of smooth muscle differentiation, was unaffected by silencing of JunB, as shown by immunoblotting (left). Quantification of immunoblots is shown in the graph (right). Gel contraction assays (D) revealed that JunB knockdown significantly reduced both basal and TGFβ1-induced changes in cellular contractility. *p<0.05, t-test (E) Inhibition of JunB inhibits basal and TGFβ1-induced contraction. This inhibition of contraction, measured quantitatively as a reduction of traction (see Methods) was statistically significant (*p<0.05, comparing siCtrl+ TGFβ1 or siJunB-TGFβ1 with siCtrl-TGFβ1; ∧p<0.05 comparing siCtrl+ TGFβ1 with siJunB+ TGFβ1 Kruskal-Wallis test). The median value of traction and the interquartile range across all tested groups is shown.