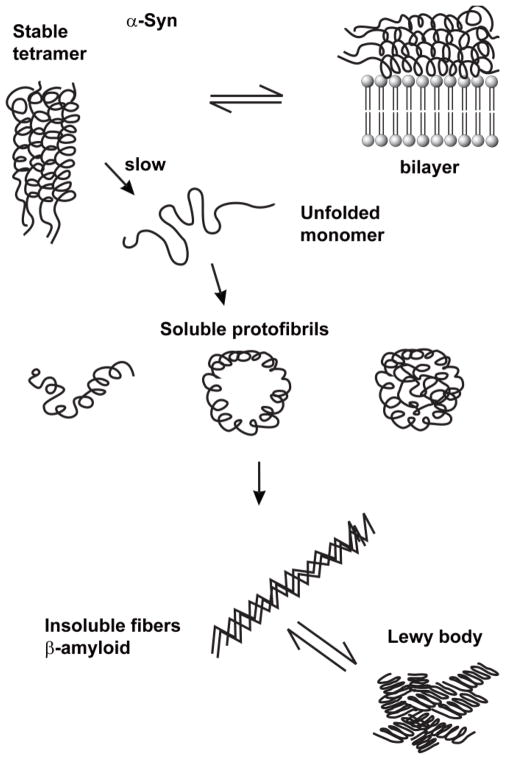
Fig. 2.
Model for α-syn aggregate/fibril formation. The α-syn tetramer is thermodynamically favored inside cells because of the high expression level of this protein. The tetramers could dissolve into unfolded monomers because of post-translational modifications, dilution, or unknown factors. Unfolded monomers can aggregate into soluble high molecular mass protofibrils and insoluble β-amyloid fibers. Emerging evidence indicates that a pathogenic conformation of α-syn is transmitted from cell-to-cell, and this transmission causes disease spread through the brain. For simplicity, in Figs. 2–4 the α-syn tetramer is depicted as a four-helix bundle where each monomer is an α-helix with a flexible tail. In the physiological α-syn tetramer, each monomer has been suggested to adopt the hair-pin-like structure shown in Fig. 1 [20].