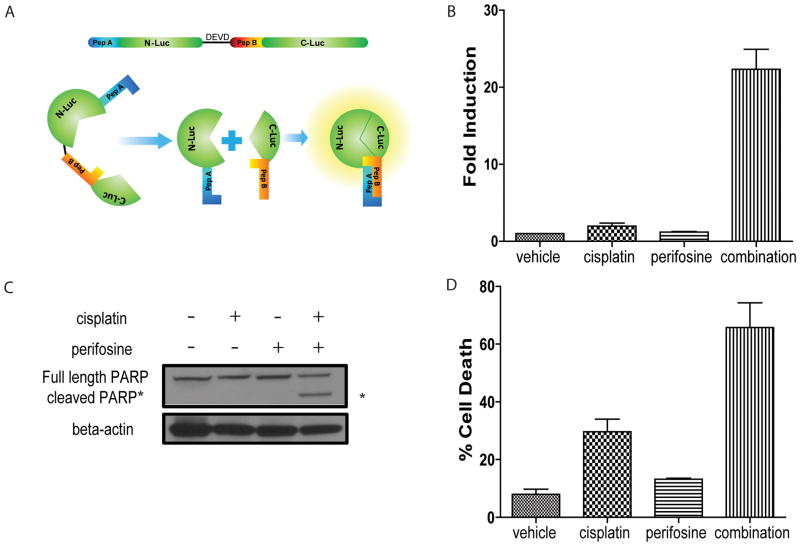
Figure 1. Perifosine sensitizes ovarian tumor cells to cisplatin-induced apoptosis.
(A) Schematic of bioluminescent apoptosis reporter molecule. Wildtype luciferase was separated into two peptides, N-terminal luciferase (N-luc) and C-terminal luciferase (C-luc). The two termini were separated by a linker region and the DEVD sequence, a recognition- and cleavage site of activated caspase-3. Two strong interacting peptides, namely peptide A and peptide B were fused to N-luc and C-luc, respectively. Upon cleavage at DEVD, peptide A and peptide B overcome the hindrance created by the linker region and reconstitute wildtype luciferase activity by bringing N-luc and C-luc in proximity. (B) Bioluminescence assay in W25-Apop cells post 20 hours treatment with PBS, cisplatin [20 μM], perifosine [30 μM] or both of perifosine and cisplatin. Fold induction of bioluminescence signals in each group was calculated at indicated time points by normalizing bioluminescence signals to the values of vehicle group. Three independent experiments were performed and data ± SEM is depicted. (C) Representative western blot for PARP in W25-Apop cells treated by cisplatin and/or perifosine for 20 hours. Cleaved PARP (89 kDa) was only observed in cells treated with combination of cisplatin and perifosine. (D) Percent cell death was assessed by PI exclusion assay of W25-Apop cells treated for 48 hours with PBS, cisplatin [20 μM], perifosine [30 μM] or both (perifosine and cisplatin). Data was obtained from three independent experiments and represent mean ± SEM.