Abstract
Considering the prevalence of 22q11.2 deletion syndrome (22q11.2 DS) of around 1:4,000 and of palatal abnormalities in 70 % of the cases of 22q11.2 DS and taking into account the Brazilian health system and its current situation of medical genetic services, this study aims to contribute to establish strategies for genetic diagnosis. The access to genetic testing at 11 services was investigated and samples from 100 patients with palatal abnormalities and suspicion of 22q11.2 DS were sent to a reference center. Laboratorial techniques included karyotyping, fluorescence in situ hybridization (FISH), and multiplex ligation-dependent probe amplification. Costs were also calculated. Disparities among centers for genetic diagnosis were evident, with remarkable regional differences. Some of the obstacles encountered were difficulties for families to show up for medical appointments, complementary evaluations, and for the clinics to send the samples to the reference center. A conclusive diagnosis was reached for 38 % of patients. Combination of karyotyping and FISH had better laboratorial cost-effectiveness. These results might represent the reality for the investigation of other genetic conditions. Clinical and laboratorial approaches herein presented could be adapted for use under different genetic conditions in the Brazilian health system, which has relatively limited financial and human resources. Suggestions for the rational implementation of genetic testing in developing countries are presented.
Keywords: 22q11 deletion, Access to genetic services, Brazil, Evaluation of genetic/genomic tools for public health, Genetic testing, Health policy
Introduction
During the last decades, an exceptional progress in genomics and biotechnology has occurred. Currently, genetics represents one of the most important fields in development (Knoppers et al. 2010). The availability of a diverse range of techniques for molecular diagnosis makes genetic testing and counseling standard practice to confirm the diagnosis of innumerous hereditary diseases (Gene Tests 2011).
Brazil is the fifth most populous country in the world. The public Brazilian health care system, called the Unified Health Care System (Sistema Único de Saúde [SUS]), serves the majority of the population. The SUS was implemented in 1990 and is based on universal and equal access to health care services and procedures, integral care, and social control (Elias and Cohn 2003).
Despite the widespread coverage of the SUS, access to genetic evaluation and counseling is restricted. This situation is similar to that of many other developing nations and reflects a dearth of clinical geneticists and genetic services in the country. Papers reviewing the situation of medical and clinical genetic services in Brazil have been published before (Marques-de-Faria et al. 2004; Horovitz et al. 2006, 2012; Castilla and Luquetti 2009).
Orofacial clefts are one of the most common congenital anomalies. They affect approximately 1 in every 600 newborns worldwide, occurring either as an isolated trait or in association with other congenital anomalies. Their impact on speech, hearing, appearance, and cognition has a prolonged and adverse influence on the health and social integration of patients, requiring multidisciplinary and multiprofessional treatment (WHO 2002).
The Reference Network for Craniofacial Treatment (Rede de Referência no Tratamento de Deformidades Craniofaciais [RRTDCF]) was created as part of the SUS to provide health care for patients with this anomaly in Brazil. The RRTDCF only offers procedures of tertiary complexity. The first evaluation of this network was conducted as part of a voluntary initiative named Cranio-Face Brazil Project (CFBP). This survey revealed the RRTDCF’s insufficient infrastructure to provide a complete and adequate genetic evaluation. Consequently, many cleft teams refer patients to other services, even for basic tests such as karyotyping (Monlleó and Gil-da-Silva-Lopes 2006). In units outside the RRTDCF, genetic evaluation and counseling is provided by untrained professionals at 30 out of 82 units (Monlleó et al. 2009).
The 22q11.2 deletion syndrome (22q11.2 DS) is the most common syndrome that has palatal anomalies as a major feature and also the most common deletion syndrome in humans. The estimated prevalence is 1:4,000 births. The prevalence of palatal anomalies among patients with 22q11.2 DS is high and the phenotype is extraordinarily variable, even within families. The diagnosis is defined by the deletion of the 22q11.2 region from chromosome 22 and fluorescence in situ hybridization (FISH) is the most commonly used technique to detect this deletion (Ruiter et al. 2003; Shprintzen 2008). However, despite the recommendation of the manufacturer of use just for research, the multiplex ligation-dependent probe amplification (MLPA) technique has been considered a rapid, reliable, economical, and high-throughput method for the diagnosis of 22q11.2 DS (Fernández et al. 2005). Considering the Brazilian health system characteristics and its current situation of medical genetic services and also the prevalence of 22q11.2 DS and palatal abnormalities, the main aim of this study was to contribute to the optimization of strategies for genetic diagnosis in Brazil. For this purpose, we investigated some critical aspects of access to genetic testing in different services and concentrated the laboratorial diagnosis of patients with palatal abnormalities and suspicion of 22q11.2 DS sent from those genetic centers, which participated voluntarily in the study. The 30-month experience of the reference center is also described.
Methods
Assessment of availability of genetic testing in the participating centers
A questionnaire was prepared comprising questions about local and regional availability of genetic testing, including karyotyping, FISH, and other molecular methods to detect microdeletions; the specific diagnosis of 22q11.2 deletion; and local infrastructure available to perform the tests using those techniques. Questions about the methods used for sending the samples to the reference center, as well as the reasons for unsent samples, were also included in the questionnaire. The questionnaires were sent by electronic mail and answered in the same way by the clinical geneticists from each center.
Sample
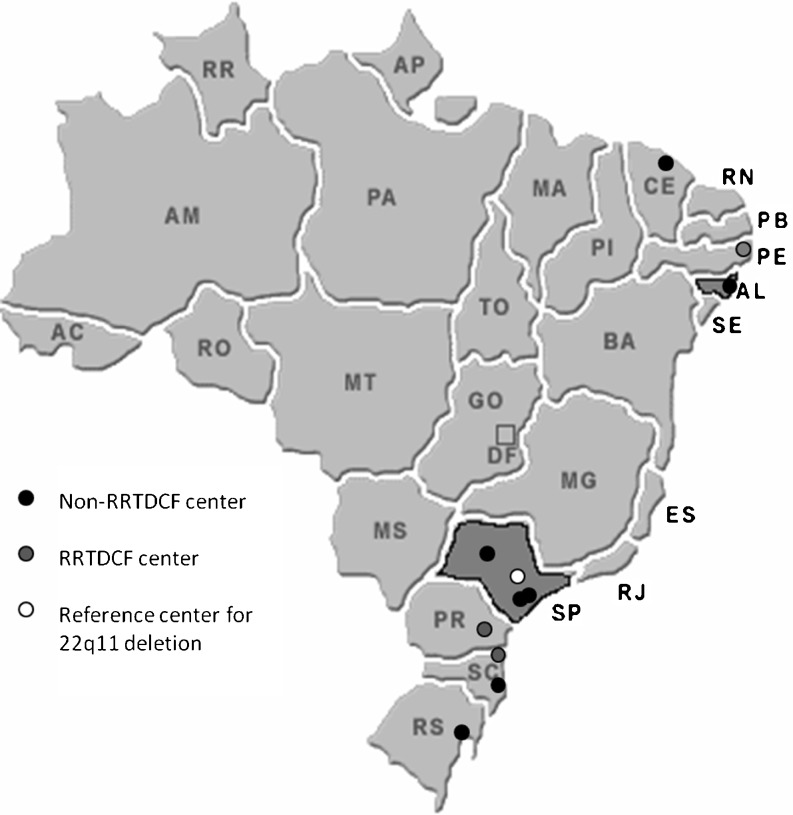
Patients with palatal abnormalities and suspicion of 22q11.2 DS from 11 different genetic centers from 3 Brazilian regions were investigated. Out of the 11 centers, 3 centers were located in the northeast, 4 in the southeast (including the reference center), and 4 in the south (Fig. 1). Three centers were RRTDCF units and the rest were genetic units which frequently received patients with palatal abnormalities and suspicion of 22q11.2 DS.
Fig. 1.
Brazil’s map showing the location of the 11 centers that sent samples and the location of the reference center
All patients were evaluated on-site by a clinical geneticist, who was responsible for evaluating the patients, sending the samples, receiving the results, and reporting them to the patients and/or patient’s families, and providing the genetic counseling. A mandatory standardized clinical protocol, including complementary investigations, was filled for each patient. This study was approved by the Research Ethics Committee from UNICAMP (protocol no. 059/2008) and a signed informed consent was obtained from all patients or their guardians.
Genetic testing
Karyotyping, with G-banding analysis, was performed on metaphase preparations of peripheral blood lymphocytes using standard techniques (resolution of 400–550 bands) in the reference center. When available, this technique was performed in the local genetic unit where the patients were attended. The FISH technique was performed using the commercially available probe TUPLE1 (Kreatech®), following the manufacturer’s instructions, with previously validated modifications. Genomic DNA was extracted from peripheral blood following standard protocols. MLPA was carried out following the manufacturer’s instructions, using the P250-A1 MLPA kit (MRC-Holland®).
Cost analysis
The reference center is a research institution (UNICAMP) with all the necessary infrastructure. The costs for the transportation of the samples were included in the budget for this study. The costs for performing each diagnostic test (karyotyping, FISH, and MLPA) were calculated including only laboratory reagents, considering the prices for purchasing it in the domestic market and transportation. Costs were initially calculated in local currency, the Brazilian real (R$) and then converted to US dollars (US$) using the exchange rate of US$1.00 = R$1.88.
Results
Availability of genetic testing in the participating centers
The availability of different techniques for genetic testing in the participating centers can be found in Table 1. The reference center was included in this analysis (center 5). The specific diagnosis of 22q11 deletion was available at five centers, one of them in the south and the rest in the southeast, during a specific period linked to research projects, but for most of them it was discontinued with the research’s interruption or conclusion. At the time the questionnaire was applied, this genetic testing was available at three centers, one on-site and two off-site.
Table 1.
Availability of different techniques for genetic diagnosis in the participant centers
| Center | Karyotyping | Microdeletion detection | ||||
|---|---|---|---|---|---|---|
| FISH | MLPA | Other molecular methods | 22q11.2 microdeletion (specifically) | |||
| Northeast | 1 | N | N | N | N | N |
| 2 | N | N | N | N | N | |
| 3 | N | N | N | N | N | |
| Southeast | 4 | Ya | Ya,b | N | Y | Ya,b |
| 5 | Ya | Ya | Ya | Ya | Ya | |
| 6 | Ya | N | Ya,b | Yb | Yb | |
| 7 | Ya | Yb | Yb | Yb | Yb | |
| South | 8 | N | N | N | N | N |
| 9 | Ya | N | N | N | Yb | |
| 10 | N | N | N | N | N | |
| 11 | Ya | Ya | Ya | N | Ya | |
FISH fluorescent in situ hybridization, MLPA multiplex ligation-dependent probe amplification, N no, Y yes
aOwn unit
bOff-site unit
Eight centers had infrastructure available to perform karyotype analysis, five had the infrastructure for FISH analysis, four for MLPA or other molecular methods that include fragment analysis by capillary electrophoresis, and four for real-time polymerase chain reaction (PCR). All these centers were genetic units. None of the RRTDCF units and no unit from the northeast region had the necessary infrastructure to perform genetic testing.
Ten services used carriers to send the samples to the reference center, seven used regular express mail, two used specialized carriers for biological sample transportation, and one used both types of carriers. Three centers had difficulties related to carrier payment and logistics: one from the northeast and two from the south region. In one center, the geneticist considered it easier to pay directly for the transportation of samples; in another center, the patients were required to pay for the transportation. At five centers, the samples of some patients could not be sent. The main reasons given were patient’s absence at the scheduled consultation, difficulties in locating previously clinically diagnosed patients, and lack of complementary exams for clinical evaluation (e.g., laryngofibroscopic examination).
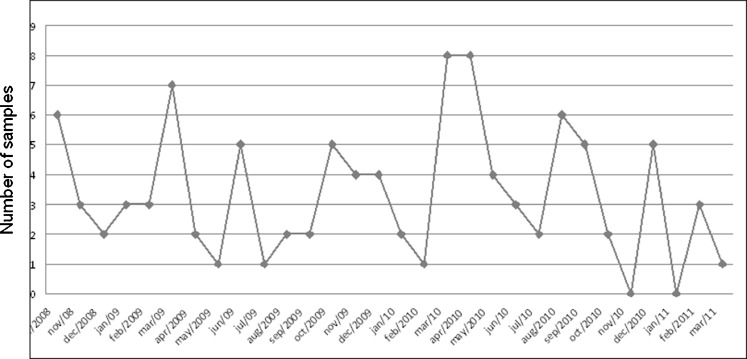
The reference center received 100 samples during 30 months. The maximum number of samples received in a month was eight and, in two of the months, no sample was received (Fig. 2). The number of samples from each region was 15 from the northeast, 35 from the southeast, and 50 from the south. The ages of the patients varied between 1 month and 33 years old (Table 2).
Fig. 2.
Number of samples sent monthly during the period of the study
Table 2.
Age of patients at diagnosis
| Age at diagnosis | Patients |
|---|---|
| 0–2 years old | 7 |
| 2–12 years old | 50 |
| 12–18 years old | 32 |
| ≥18 years old | 11 |
Chromosomal abnormalities
The karyotype analysis was performed for 93 out of 100 patients. For 62 patients, this investigation was performed at the reference center, including the patients evaluated at that unit; patients from the three centers located in the northeast and one center in the south. In seven cases, karyotyping was not performed at the local genetic unit, and no samples collected appropriately for lymphocyte cultures were sent to the reference center.
Chromosome abnormalities were found in six patients. In two of them, it was not considered causative of the phenotype. Therefore, chromosome abnormality was related to the phenotype in only four patients, with only one of them involving 22q11.2 deletion.
22q11.2 deletion
MLPA and FISH were performed for 78 cases and MLPA only for 22 cases. Microdeletion in 22q11.2 was detected in 35 patients, none of which had atypical deletions. Screening by MLPA for 22q11.2 deletion was carried out for parents in 13 cases in which the child was found to have 22q11.2 deletion, according to the interest expressed by the families. Deletion was not found in any of the parents who were analyzed. In one patient, the MLPA technique detected 8p23.1 duplication, but there was no abnormality in the 22q11 region. This patient also carried another chromosome abnormality detected by conventional chromosome analysis. Considering the results from karyotyping, FISH, and MLPA, a conclusive diagnosis was reached for 38 % of the sample.
Cost analysis
The cost for karyotype analysis per individual, considering only laboratory reagents and transportation of the sample, was US$34.43; for FISH, including the lymphocyte culture, it was US$137.61 using the manufacturer’s recommended protocol and US$74.59 using the adapted protocol; and for MLPA, including DNA extraction, it was US$84.16. Human resources and equipment costs were not included in the calculation.
Discussion
In Brazil, birth defects have represented the second most common cause of infant mortality during the last decade (Brasil Ministério da Saúde 2011). Unfortunately, the application of advances in medical genetics in the form of genetic services in developing nations is accessible only to the wealthy and educated. Thus, the integration of genetic health care in the developing world is an important goal in the minimization of health care disparities (Daar et al. 2007).
Besides difficulties with genetic evaluation, laboratorial investigations in this area are also limited in Brazil. Although karyotyping is the only genetic test provided by public services, it is available only in few laboratories, usually those linked to universities, with limited human resources. Only some laboratories are equipped for high-resolution chromosomal analysis, FISH, and DNA testing (Marques-de-Faria et al. 2004; Horovitz et al. 2006). Private laboratories provide karyotyping, FISH, and molecular analyses for various genetic diseases, but only a minority of the Brazilian population can afford private health insurance or direct payment for genetic testing (WHO 2006).
A report about public policies in clinical genetics in Brazil pointed out that, for a functional and economic laboratory network, the implementation of specialized laboratories in all genetic services or even in all Brazilian states would not be justifiable, suggesting that basic laboratories, such as cytogenetic laboratories, could be implemented regionally or at a state level, and more specialized analyses could be offered by a few reference centers in the country (Horovitz et al. 2006).
The present study offers a comprehensive portrayal of a specific situation in this country and the solutions proposed could be used to start planning a national approach for genetic testing in the Brazilian public health care system. The 22q11.2 DS associated with palatal abnormalities was chosen in view of a previous study showing the need for genetic testing in craniofacial hospitals (Monlleó and Gil-da-Silva-Lopes 2006), the prevalence of this condition among cleft palate individuals (Ruiter et al. 2003), and the existence of a voluntary clinical genetics network on craniofacial anomalies (CFBP).
It was found that genetic testing was available for part of the participant centers of this study; however, the inequity among the different regions of the country was clear. None of the units in the northeast region had the necessary infrastructure to perform genetic testing (Table 1). The unequal access to public health services and medical genetic services in Brazil has already been reported in medical literature (Marques-de-Faria et al. 2004; Travassos et al. 2006; Horovitz et al. 2012). In the northeast region, genetic services are mainly clinical, and in some states, there is no local laboratory (Horovitz et al. 2012). This scenario reflects the socioeconomic development discrepancy between the north/northeast and the south/southeast regions, with greater investments in public health having gone into the south/southeast regions. Based on the extensive differences in genetic service infrastructure, suggestions for each region will be presented separately at the end of this paper.
Difficulties in sending the samples to the reference center were observed for half of the participating centers. In view of these difficulties, only a small number of samples were sent each month. Notably, only 15 of the samples came from the northeast region. Among the many reasons for that would be the limited access to a clinical geneticist and complementary examinations, as mentioned by other researchers (Marques-de-Faria et al. 2004; Horovitz et al. 2006; Fontes et al. 2012). Again, this situation reflects the socioeconomic discrepancy among the Brazilian regions. The gross domestic product per capita for the northeast region is US$4,344, while for the south and southeast regions, it is US$10,278 and US$11,780, respectively (R$1.88 = US$1.00; reference year 2009) (IBGE 2010). Therefore, the results observed in this study were expected and point to the need for improvement in the general basic structure of the public health care system in Brazil, especially in the northeast region. This is also evidenced by the absence of participating centers from the midwest and north regions. The main reason for this is extreme limitations in the genetics services offered and the absence of cleft teams available during this study.
Considering the prevalence of orofacial clefts and 22q11.2 DS (Ruiter et al. 2003) and a Brazilian birthrate of around 2,800,000 live births (Brasil Ministério da Saúde 2010), an incidence of approximately 5,000 new cases of orofacial clefts and 700 new cases of 22q11.2 DS would be expected each year. In this 30-month study, samples from 100 patients from 11 different centers, including 3 reference centers for craniofacial anomalies, were received. Out of the 100 patients, 43 were older than 12 years old. Considering that 70 % of 22q11.2 DS patients present palatal abnormalities (McDonald-McGinn and Sullivan 2011), these findings point to an underdiagnosis of patients with 22q11.2 DS, which in turn reflects an error in the management of patients with craniofacial defects, especially in the northeast, the region with the fewer number of patients included in this study.
On the other hand, the small monthly number of samples sent justifies the maintenance of reference centers. This strategy allows a rational and economic use of infrastructure and human resources, improving the technical quality and reducing the expenses for the tests. These suggestions are in accordance with a previous study carried out in Brazil (Horovitz et al. 2006).
Difficulties for sample transportation were observed at some centers, including centers from the northeast and south regions, which indicate that problems with sample transportation are not only due to financial reasons, but also to logistics problems, which needs to be improved and regulated in all country regions.
In relation to the age groups, it was observed that 43 % of the patients were older than 12 years old. Even considering that the clinical heterogeneity of 22q11.2 DS hampers the clinical diagnosis, there was a delay in the diagnosis for many of the patients. That could be also related to limited access to genetic evaluation and, even with the suspicion of 22q11.2 DS, there was likely no possibility to perform a laboratorial test before. The advanced age in diagnosis for cleft individuals was also noted in another study of the CFBP (Fontes et al. 2012).
Chromosomal abnormalities not related to the 22q11 region were detected in 3 % of the cases, which corroborates the utility of karyotype analysis for those cases. 22q11.2 deletion was found in 35 % of the cases. When the combined approach of karyotyping and FISH or MLPA was used, a conclusive diagnosis was reached for 38 % of the sample. This number is a little higher than what was found in other studies with different inclusion criteria (Bartsch et al. 2003; Katzman et al. 2005; Brunet et al. 2006; Oh et al. 2007). Therefore, it is conceivable that genetic evaluation before laboratorial tests could lead to a greater number of positive cases.
The clinical data were collected using a standardized clinical protocol and were then sent to the reference center. This approach would be useful also for collecting specific information about positive cases and to review the clinical criteria to perform genetic testing in the public health care system. That information would lead to improved reference and the reduction of unnecessary genetic testing. The need for this kind of data collection and a review of genetic testing is one of recommendations of the Human Variome Project (Kohonen-Corish et al. 2010).
FISH has been considered a prohibitively expensive technique in developing countries (Jehee et al. 2011). The estimated costs for the techniques used in this study, considering only laboratory reagents and transportation, showed that MLPA and FISH are not so expensive or prohibitive for implementation in the public health care system. However, since the FISH technique is locus-specific, it may not be efficient for atypical deletion, which represents 3 % of the cases (Emanuel 2008). However, the combination of karyotyping and FISH allows detecting more information for each patient, which would justify choosing this option.
MLPA and FISH were performed for most of the patients, allowing a comparison between the techniques. However, for routine genetic testing, using just one technique would normally be enough. MLPA has the advantage of permitting to test several loci at the same time and being able to detect atypical deletions in 22q11.2, as well as other microdeletions. However, this technique is not appropriate for chromosomal investigations in general. A significant disadvantage is the need to test at least ten samples together to reduce the costs since five normal controls are required in each reaction. This characteristic of MLPA increases the turnaround time for results. Besides, MLPA requires the use of automated DNA analyzers, which are expensive to acquire and to maintain.
The equipment already in operation at the laboratory, the human resources required, and the technical support involved are important points to consider for the implementation of diagnostic tests. In the present study, the utilization of an adapted protocol for FISH reduced the reagents cost for this technique from US$137.61 to US$42.22. Although this technique requires expensive equipment, it is not expensive to maintain. Furthermore, the adaptation of the protocol in this study seems to be a good way to reduce costs and make this technique feasible for implementation in developing countries.
Real-time PCR, analysis of polymorphic markers, and array comparative genomic hybridization (a-CGH) also test the 22q11.2 deletion. In the USA, a-CGH has been adopted for the diagnosis of 22q11.2 DS, but it is not adequate for the Brazilian situation of limited resources. Other PCR-based techniques have been used for research purposes, but they are not so reliable for diagnostic tests (Yang et al. 2009; McDonald-McGinn and Sullivan 2011). Nevertheless, in developing countries, improvement of PCR-based techniques and its implementation for clinical diagnosis could be a cost-effective strategy if infrastructure were available. However, using this approach, chromosomal abnormalities would not be detected. Therefore, karyotyping should always be part of the diagnostic tools.
In Brazil, the only genetic test currently available in the SUS for congenital defects investigation is karyotyping, which has coverage limited to US$18.00 (R$1.88 = US$1.0) for each test. The implementation of FISH or MLPA would increase the costs of the testing by more than two or three times when compared with karyotyping. However, karyotyping is not able to detect microdeletions, so the inclusion of FISH or MLPA is warranted.
Most of the public clinical genetic services in Brazil are linked to universities and research centers. In this way, the association of research and clinical investigation has permitted public access to good quality genetic services in many regions of the country. Also, the use of research grants for implementing new technologies for molecular analyses has permitted the diagnosis of patients enrolled in research projects. This association is a good use of resources already established and could be a possible path to start the implementation of genetic testing in the public health care system. For these reasons, the strategy herein proposed used the structure of a university center, reducing the need for investment in equipment. The human resources involved in this study were also from the institution since they were all postgraduate students. For a large-scale implementation, the SUS would have to invest in specialized human resources.
The strategy adopted in this study, which was to use the infrastructure that already existed in a genetic service linked to a university and concentrating the testing for a specific syndrome in patients from different clinical services, could be a good example of how to implement genetic testing for public genetic services in Brazil. However, the availability of appropriate laboratory testing depends on the inclusion of new tests in the list of procedures covered by the SUS. Genetic testing for the diagnosis of well-known syndromes, such as 22q11.2 DS, are not considered as research anymore, and the costs should be transferred from the realm of research to health assistance.
Efforts from government and genetic professionals are necessary for the effective incorporation of new genetic testing in the SUS. Establishing standard protocols for specific syndromes, defining the costs of each procedure, as well as organizing a network and logistics for patient’s referral/counter-referral and to transport samples are necessary initiatives to start implementing these procedures. Besides this, improvements and expansions to the clinical genetic services, especially in the north/northeast regions, are also necessary.
The findings of this study portray the situation of genetic testing for 22q11.2 deletion in the public health care system in Brazil. The conclusions drawn from these findings could also be applied to other genetic conditions. The implementation of other investigation approaches, aiming to identify critical regions for the installation of reference centers, possibilities to reduce costs to make diagnosis, including technical adaptations, could bring other perspectives to the rational implementation of genetic testing in the public health care system in Brazil and other developing countries. This study also makes some suggestions regarding genetic tests in Brazil:
Education about genetics for all health professionals, including changing educational curricula of medical schools;
Education of physicians from different specialties for use of genetic testing, including education about the limitations and risks;
Increase in the number of clinical geneticists, especially in the north/northeast regions;
Implementation of a national laboratory network for genetic testing using the infrastructure that already exists in universities/research centers and concentrating specific diagnosis, such as microdeletion syndromes;
Implementation of genetic laboratories in the north/northeast regions to provide basic genetic tests, such as karyotyping1, and in the long term expand to allow more specific diagnosis, such as the diagnosis of microdeletion syndromes. Such specific diagnosis could be concentrated in just a few laboratories;
Mapping of the genetic laboratories in the south/southeast regions, which could provide support and perform microdeletion diagnosis for other genetic centers, including centers from the north/northeast regions, thus optimizing the existing infrastructure;
Formalization of the referral of samples between the genetic centers;
Establishment of minimal requirements for reference centers. It would be preferable to adapt and improve centers which already have the necessary infrastructure and human resources available;
Establishment of official mechanisms for sample transportation respecting regulatory issues in Brazil;
Establishment of a working group for the development and dissemination of standards for routines and quality control for genetic laboratories, as well as the performance of cost-effectiveness studies of different techniques, aiming to implement good quality tests with good cost-effectiveness;
Participation of a clinical geneticist before and after genetic testing to guarantee the rational and ethical use of genetic tests and also genetic counseling;
Establishment of research centers that could provide support for special cases, including laboratorial support and discussion and orientation about results;
Creation of a national database that includes epidemiological data collection and genetic testing.
Acknowledgments
The authors are grateful to the patients and their families, and to all the participant clinical geneticists and institutions. This study was supported with funding from the CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico (# 01300.000433/2008-00 and #502438/2010-0), FAPESP - Fundação de Amparo a Pesquisa do Estado de São Paulo (#2008/50421-4, # 2008/096574; # 2009/09507-5 and 2009/08756-1) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
As part of the strategy adopted by the CFBP in 2009, a Cytogenetics Laboratory was implemented in the northeast (Universidade Estadual de Ciências da Saúde de Alagoas [UNCISAL], Maceio, Alagoas), with support by CNPq.
References
- Bartsch O, Nemecková M, Kocárek E, Wagner A, Puchmajerová A, Poppe M, Ounap K, Goetz P. DiGeorge/velocardiofacial syndrome: FISH studies of chromosomes 22q11 and 10p14, and clinical reports on the proximal 22q11 deletion. Am J Med Genet. 2003;A117A(1):1–5. doi: 10.1002/ajmg.a.10914. [DOI] [PubMed] [Google Scholar]
- Brasil Ministério da Saúde (2010) SINASC (Sistema de Informações sobre nascidos vivos). Available at http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinasc/cnv/nvuf.def. Accessed August 2012
- Brasil Ministério da Saúde (2011) Coordenação Geral de Informações e Análises Epidemiológicas. Painel de Monitoramento da Mortalidade Infantil e Fetal. Available at http://svs.aids.gov.br/dashboard/mortalidade/infantil.show.mtw. Accessed 3 November 2011
- Brunet A, Gabau E, Perich RM, Valdesoiro L, Brun C, Caballin MR, Guitart M. Microdeletion and microduplication 22q11.2 screening in 295 patients with clinical features of DiGeorge/velocardiofacial syndrome. Am J Med Genet. 2006;140A:2426–2432. doi: 10.1002/ajmg.a.31499. [DOI] [PubMed] [Google Scholar]
- Castilla EE, Luquetti DV. Brazil: public health genomics. Public Health Genomics. 2009;12:53–58. doi: 10.1159/000153424. [DOI] [PubMed] [Google Scholar]
- Daar AS, Berndtson K, Persad DL, Singer PA. How can developing countries harness biotechnology to improve health? BMC Public Health. 2007;7:346. doi: 10.1186/1471-2458-7-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PEM, Cohn A. Health reform in Brazil: lessons to consider. Am J Public Health. 2003;93:44–48. doi: 10.2105/AJPH.93.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel BS. Molecular mechanisms and diagnosis of chromosome 22q11.2 rearrangements. Dev Disabil Res Rev. 2008;14(1):11–18. doi: 10.1002/ddrr.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández L, Lapunzina P, Arjona D, López Pajares I, García-Guereta L, Elorza D, Burgueros M, De Torres ML, Mori MA, Palomares M, García-Alix A, Delicado A. Comparative study of three diagnostic approaches (FISH, STRs and MLPA) in 30 patients with 22q11.2 deletion syndrome. Clin Genet. 2005;68(4):373–378. doi: 10.1111/j.1399-0004.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- Fontes MB, Almeida LN, Reis-Junior GO, Vieira-Filho IJ, Santos KM, Anjos FS, Andrade AK, Porciuncula CG, Oliveira MC, Pereira RM, Vieira TA, Viguetti-Campos NL, Gil-da-Silva-Lopes VL, Monlleo IL (2012) Local strategies to address health needs of individuals with oral clefts in Alagoas, Brazil. Cleft Palate Craniofac J. doi:10.1597/11-069 [DOI] [PubMed]
- Gene Tests (2011) Available at http://www.ncbi.nlm.nih.gov/sites/GeneTests/. Accessed December 2011
- Horovitz DD, Cardoso MH, Llerena JC, Jr, de Mattos RA. Birth defects in Brazil and health care: proposals for public policies in clinical genetics. Cad Saude Publica. 2006;22(12):2599–2609. doi: 10.1590/S0102-311X2006001200010. [DOI] [PubMed] [Google Scholar]
- Horovitz DD, de Faria Ferraz VE, Dain S, Marques-de-Faria AP (2012) Genetic services and testing in Brazil. J Community Genet (in press) [DOI] [PMC free article] [PubMed]
- IBGE (2010) Instituto Brasileiro de Geografia e Estatística. Produto Interno Bruto a preços correntes e Produto Interno Bruto per capita segundo as Grandes Regiões, as Unidades da Federação e os municípios—2005–2009. Available at http://www.ibge.gov.br/home/estatistica/economia/pibmunicipios/2005_2009/tabelas_pdf/tab01.pdf. Accessed August 2012
- Jehee FS, Takamori JT, Medeiros PF, Pordeus AC, Latini FR, Bertola DR, Kim CA, Passos-Bueno MR. Using a combination of MLPA kits to detect chromosomal imbalances in patients with multiple congenital anomalies and mental retardation is a valuable choice for developing countries. Eur J Med Genet. 2011;54(4):e425–e432. doi: 10.1016/j.ejmg.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Katzman PJ, Wang B, Sawhney M, Wang N. Differential detection of deletion 22q11.2 syndrome by specialty and indication. Pediatr Dev Pathol. 2005;8(5):557–567. doi: 10.1007/s10024-005-0056-1. [DOI] [PubMed] [Google Scholar]
- Knoppers BM, Leroux T, Doucet H, Godard B, Laberge C, Stanton-Jean M, Fortin S, Cousineau J, Monardes C, Girard N, Levesque L, Durand C, Farmer Y, Dion-Labrie M, Bouthillier ME, Avard D. Framing genomics, public health research and policy: points to consider. Public Health Genomics. 2010;13(4):224–234. doi: 10.1159/000279624. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish MR, Al-Aama JY, Auerbach AD, Axton M, Barash CI, Bernstein I, Béroud C, Burn J, Cunningham F, Cutting GR, den Dunnen JT, Greenblatt MS, Kaput J, Katz M, Lindblom A, Macrae F, Maglott D, Möslein G, Povey S, Ramesar R, Richards S, Seminara D, Sobrido MJ, Tavtigian S, Taylor G, Vihinen M, Winship I, Cotton RG, Human Variome Project Meeting How to catch all those mutations—the report of the third Human Variome Project Meeting, UNESCO Paris. Hum Mutat. 2010;31(12):1374–1381. doi: 10.1002/humu.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-de-Faria AP, Ferraz VE, Acosta AX, Brunoni D. Clinical genetics in developing countries: the case of Brazil. Community Genet. 2004;7(2–3):95–105. doi: 10.1159/000080777. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine (Baltimore) 2011;90(1):1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- Monlleó IL, Gil-da-Silva-Lopes VL. Brazil’s Craniofacial Project: genetic evaluation and counseling in the Reference Network for Craniofacial Treatment. Cleft Palate Craniofac J. 2006;43(5):577–579. doi: 10.1597/04-203. [DOI] [PubMed] [Google Scholar]
- Monlleó IL, Mossey PA, Gil-da-Silva-Lopes VL. Evaluation of craniofacial care outside the Brazilian reference network for craniofacial treatment. Cleft Palate Craniofac J. 2009;46(2):204–211. doi: 10.1597/07-153.1. [DOI] [PubMed] [Google Scholar]
- Oh AK, Workman LA, Wong GB. Clinical correlation of chromosome 22q11.2 fluorescent in situ hybridization analysis and velocardiofacial syndrome. Cleft Palate Craniofac J. 2007;44(1):62–66. doi: 10.1597/05-192. [DOI] [PubMed] [Google Scholar]
- Ruiter EM, Bongers EM, Smeets DF, Kuijpers-Jagtman AM, Hamel BC. No justification of routine screening for 22q11 deletions in patients with overt cleft palate. Clin Genet. 2003;64(3):216–219. doi: 10.1034/j.1399-0004.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 years of study. Developmental Disabilities. 2008;14:3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos C, de Oliveira EXG, Viacava F. Desigualdades geográficas e sociais no acesso aos serviços de saúde no Brasil: 1998 e 2003. Cien Saude Colet. 2006;11(4):975–986. doi: 10.1590/S1413-81232006000400019. [DOI] [Google Scholar]
- WHO—World Health Organization (2002) Global strategies to reduce the health-care burden of craniofacial anomalies. WHO, Geneva. Available at http://www.who.int/genomics/about/en/cfa1-3.pdf
- WHO—World Health Organization (2006) Medical genetic services in developing countries. The ethical, legal and social implications of genetic testing and screening. Human Genetics, Chronic Diseases and Health Promotion, WHO, Geneva. Available at http://www.who.int/genomics/publications/GTS-MedicalGeneticServices-oct06.pdf
- Yang C, Zhu X, Yi L, Shi Z, Wang H, Hu Y, Wang Y. Comparative study of three PCR-based copy number variant approaches, CFMSA, M-PCR, and MLPA, in 22q11.2 deletion syndrome. Genet Test Mol Biomarkers. 2009;13(6):803–808. doi: 10.1089/gtmb.2009.0058. [DOI] [PubMed] [Google Scholar]