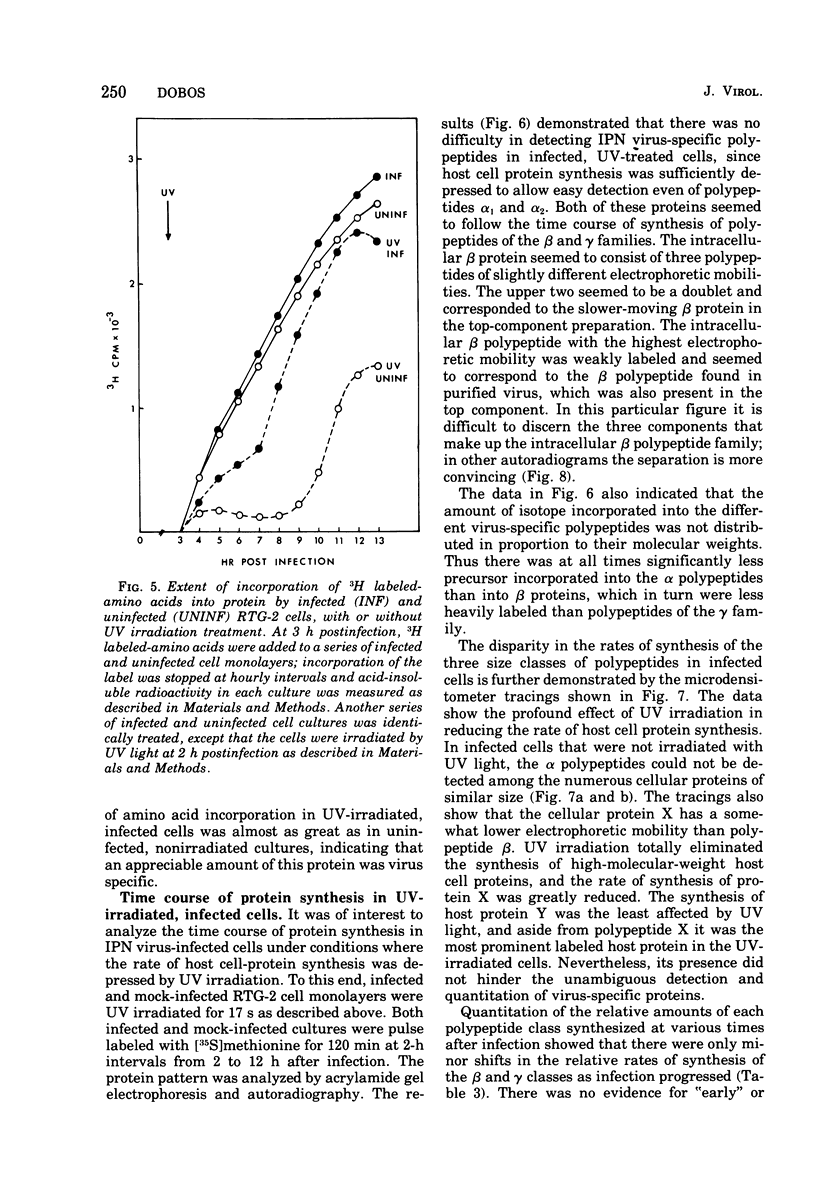
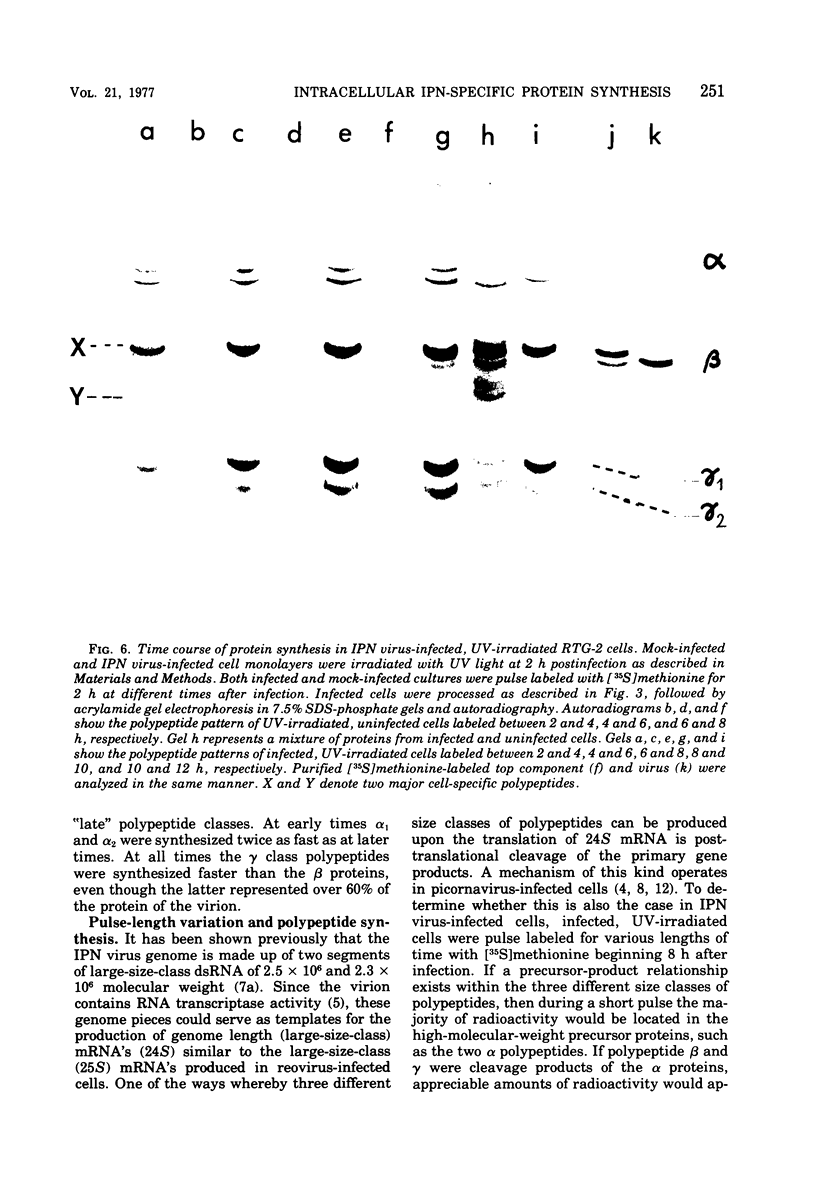
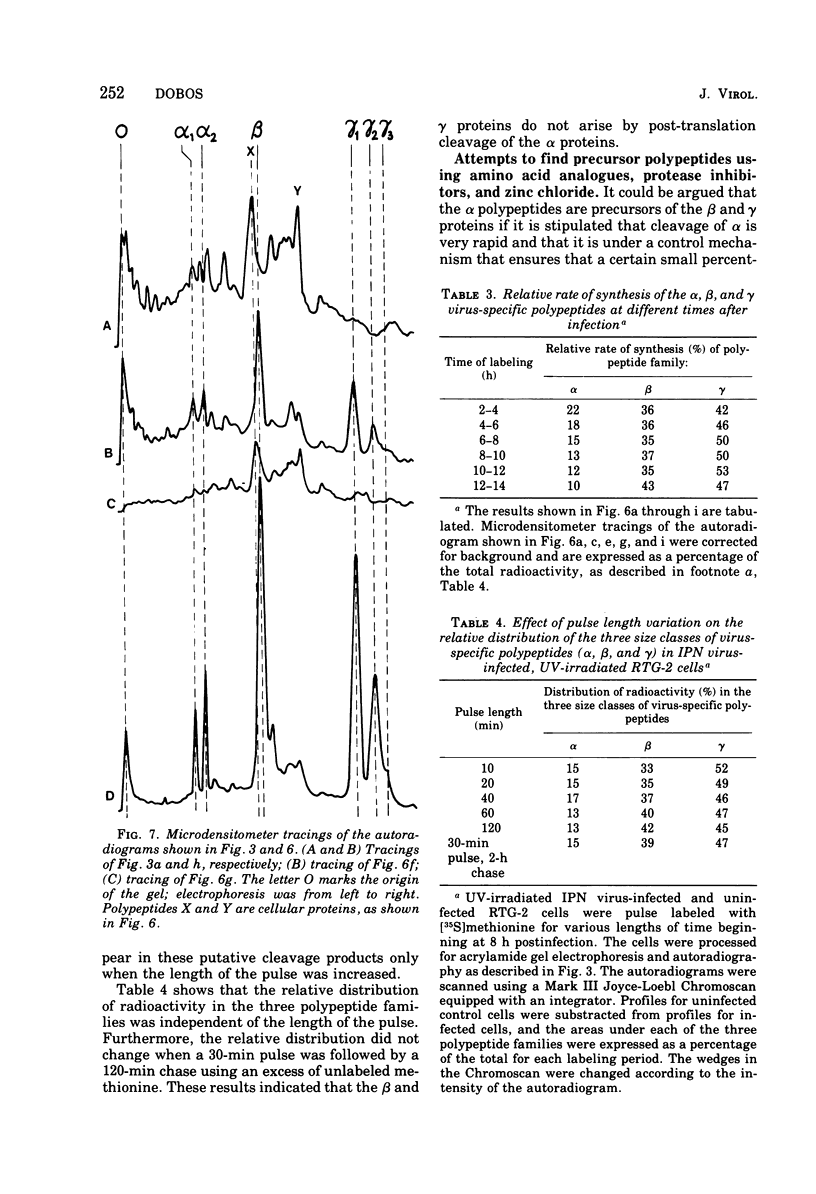
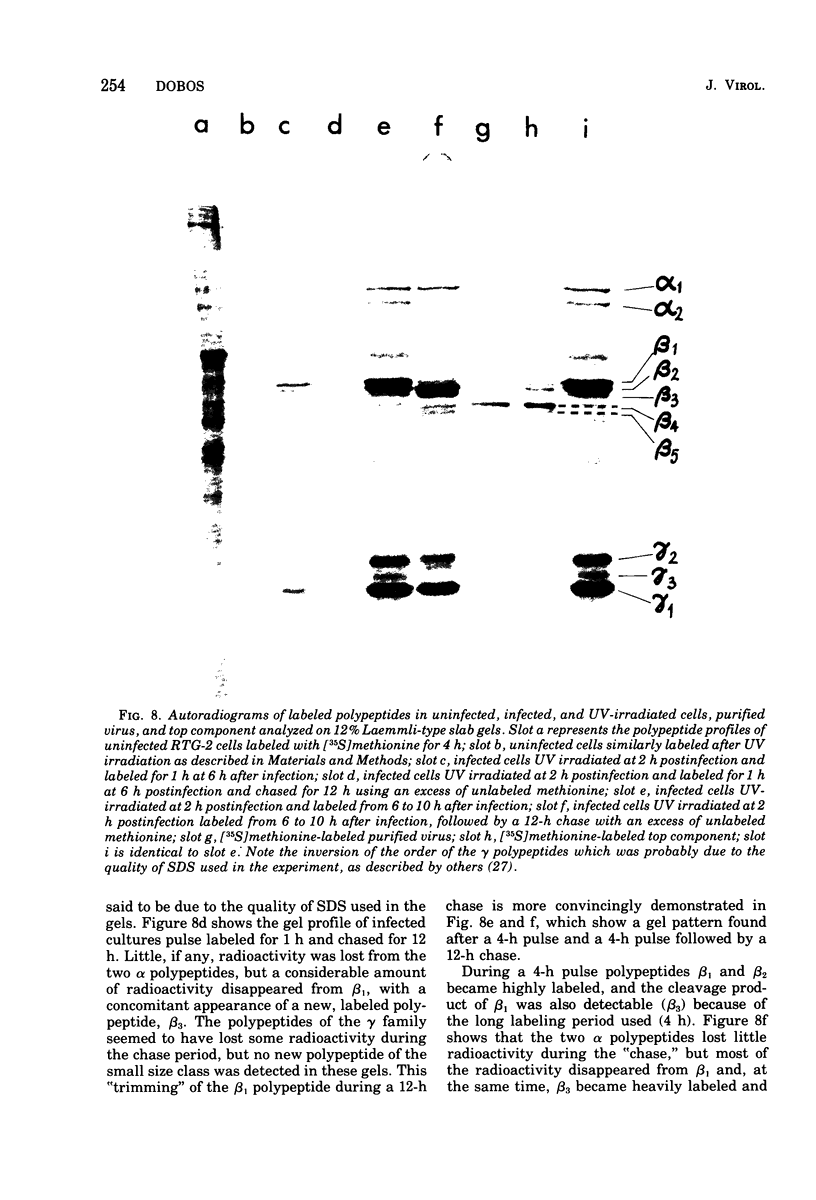
Abstract
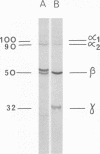
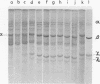
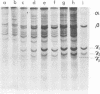
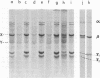
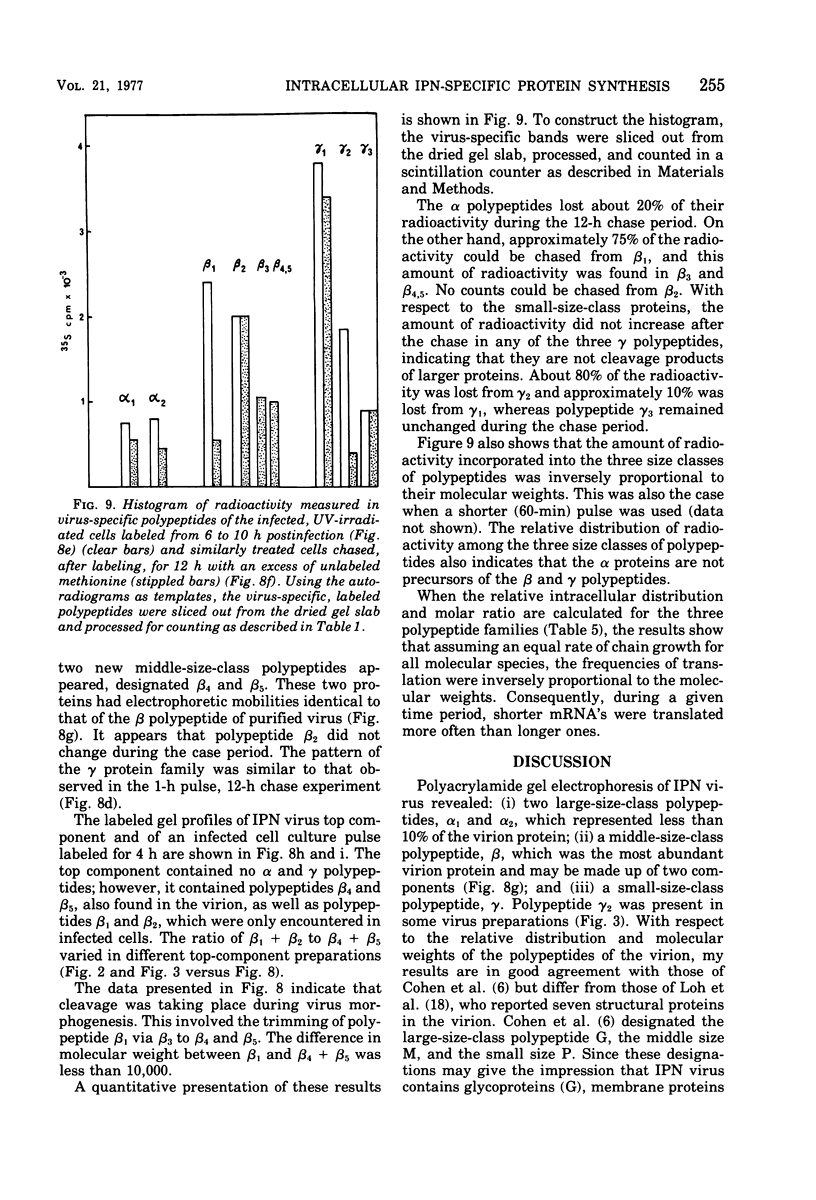
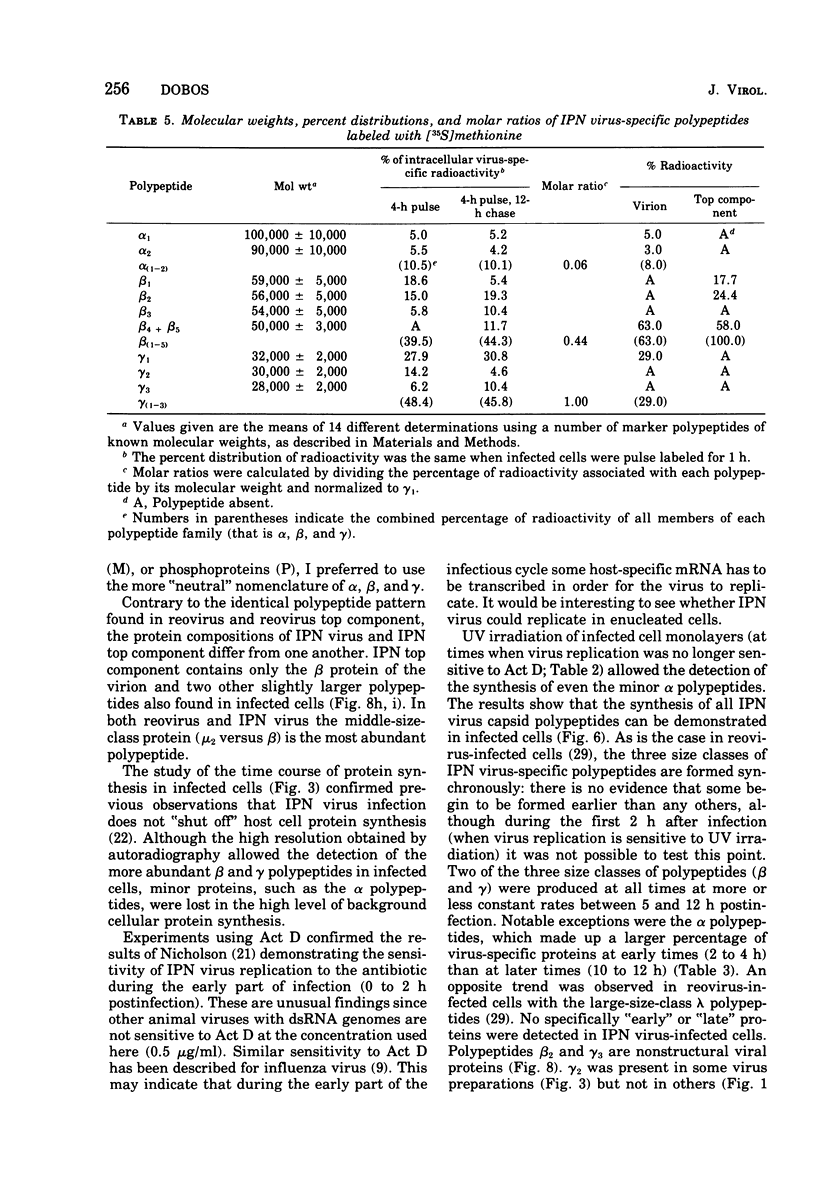
A study of virus-specific protein synthesis in infectious pancreatic necrosis virus-infected RTG-2 cells was undertaken to find a relationship between the coding capacity of virus genome (two segments of double-stranded RNA of 2.5 X10(6) and 2.3 X 10(6) molecular weight) and the sizes and relative amounts of polypeptides in the virion and in infected cells. The time course of virus-specific protein synthesis was followed by pulse labeling infected UV-irradiated cells with [35S]methionine and analyzing the labeled proteins by polyacrylamide gel electrophoresis followed by autoradiography. Three size classes of virus-specific polypeptides were synthesized, in the same relative proportion, throughout the infectious cycle, beginning 3 h postinfection. Their designation and molecular weight was as follows: alpha1, 1000,000; alpha2, 90,000; beta1, 59,000; beta2, 56,000; gamma1, 32,000; gamma2, 30,000; and gamma3, 28,000. Experiments using amino acid analogues, protease inhibitors, ZnCl2, and supraoptimal temperatures showed that polypeptides of the beta and gamma families did not arise from the alpha polypeptides by post-translational cleavage. Slow cleavage late in the infectious cycle could be demonstrated, since during 12-h period radioactivity was chased from beta1 via beta3 to beta4 (molecular weight 50,000) and beta5 (molecular weight, 49,000). During the chase most of gamma2 was degraded, whereas radioactivity could not be chased from the remaining virus-specific polypeptides. Purified virus contained polypeptides alpha1, alpha2, beta4, beta5, and gamma1. The beta polypeptides made up over 60% of the virion proteins. The results suggest that infectious pancreatic necrosis vibrus possesses a unique mechanism for synthesis of three size-classes of proteins using mRNA transcripts from two high-molecular-weight double-stranded RNA genome segments.
Full text
PDF


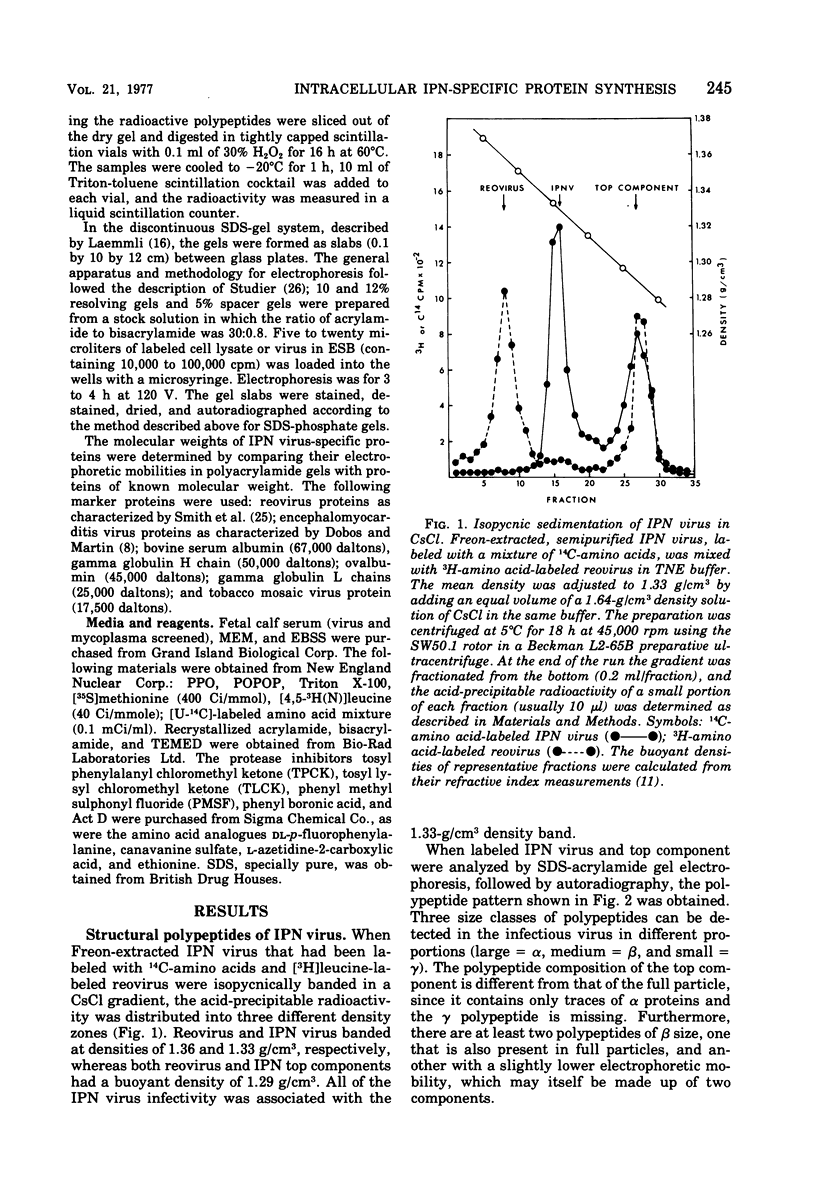
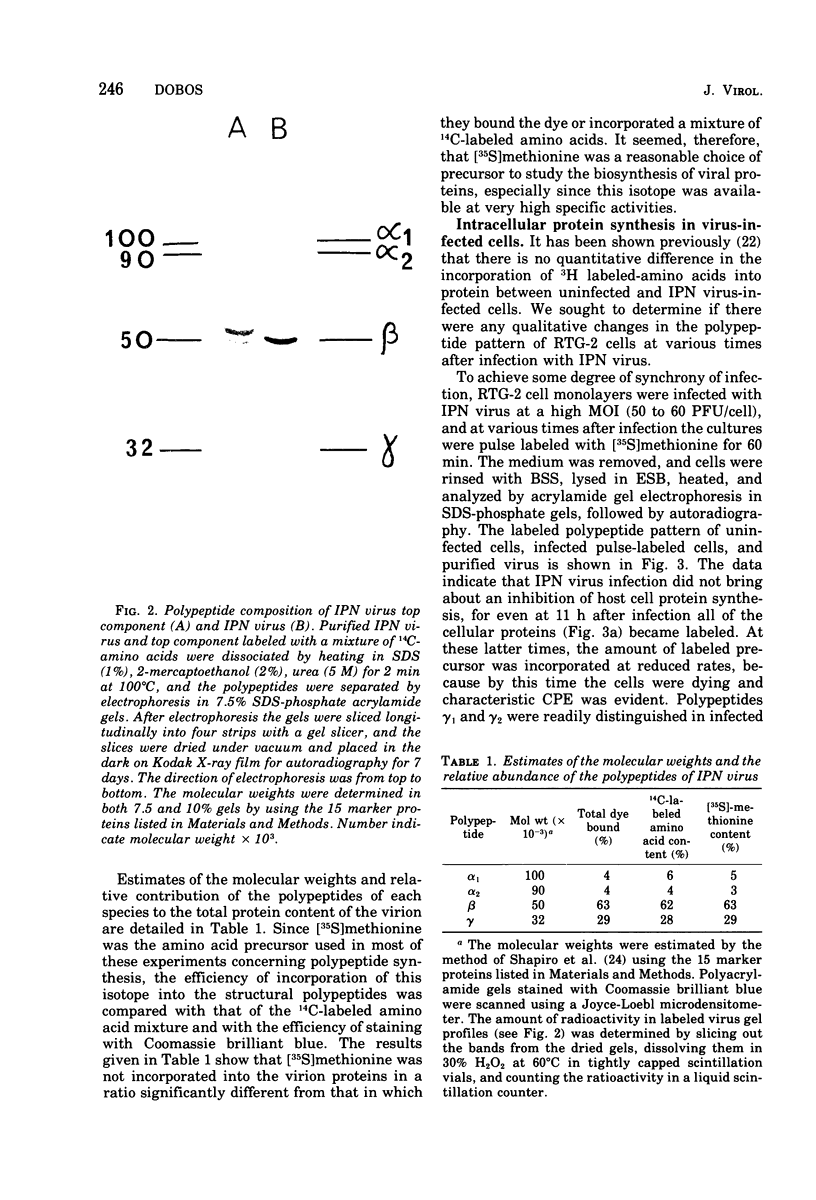

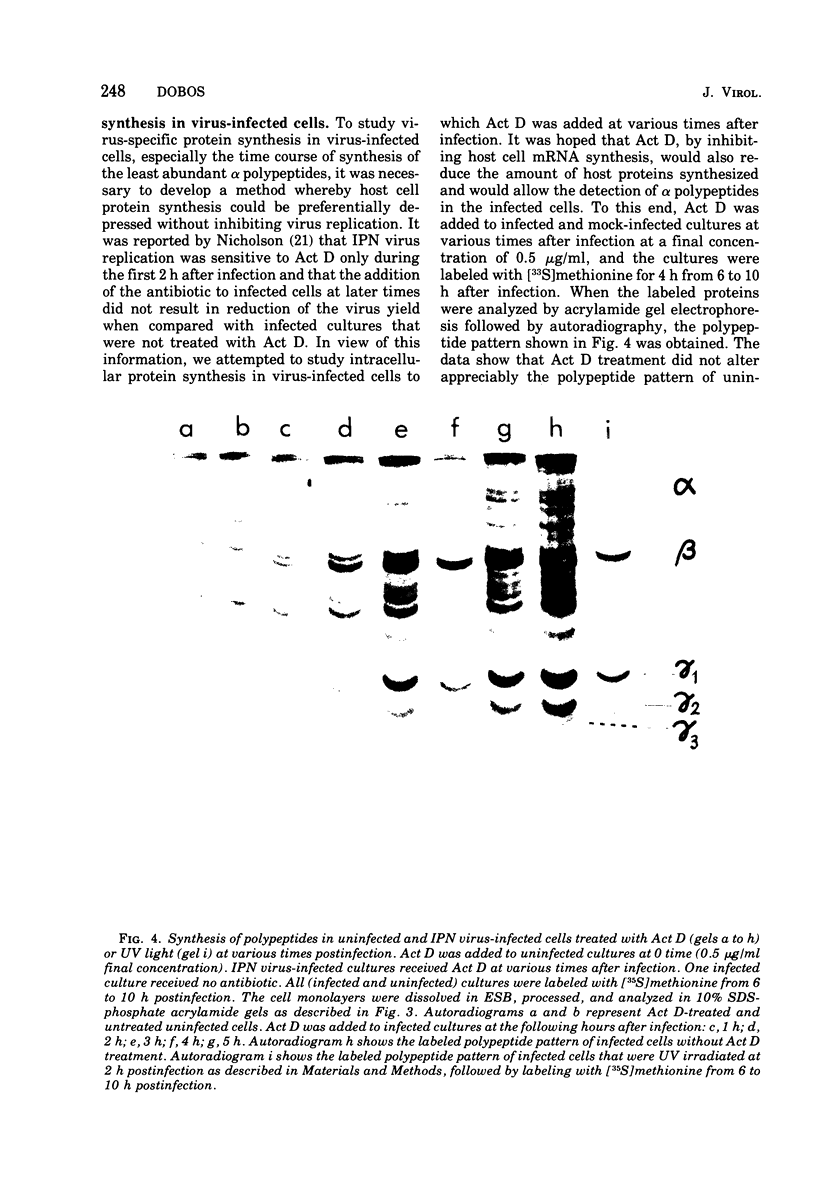




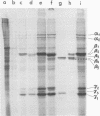
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Poinsard A., Scherrer R. Physico-chemical and morphological features of infectious pancreatic necrosis virus. J Gen Virol. 1973 Dec;21(3):485–498. doi: 10.1099/0022-1317-21-3-485. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucletic acid polymerase activity in purified infectious pancreatic necrosis virus of trout. Biochem Biophys Res Commun. 1975 Feb 3;62(3):689–695. doi: 10.1016/0006-291x(75)90454-4. [DOI] [PubMed] [Google Scholar]
- Dobos P., Martin E. M. Virus-specific polypeptides in ascites cells infected with encephalomyocarditis virus. J Gen Virol. 1972 Nov;17(2):197–212. doi: 10.1099/0022-1317-17-2-197. [DOI] [PubMed] [Google Scholar]
- Dobos P. Size and structure of the genome of infectious pancreatic necrosis virus. Nucleic Acids Res. 1976 Aug;3(8):1903–1924. doi: 10.1093/nar/3.8.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Use of gum tragacanth overlay, applied at room temperature, in the plaque assay of fish and other animal viruses. J Clin Microbiol. 1976 Mar;3(3):373–375. doi: 10.1128/jcm.3.3.373-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P. P., Walters C. P. Influenza virus-induced ribonucleic acid nucleotidyltransferase and the effect of actinomycin D on its formation. Biochemistry. 1966 Jan;5(1):231–235. doi: 10.1021/bi00865a030. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Electron microscopical and biochemical characterization of infectious pancreatic necrosis virus. J Virol. 1972 Oct;10(4):824–834. doi: 10.1128/jvi.10.4.824-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Replication of IPN virus: a cytochemical and biochemical study in SWT cells. Proc Soc Exp Biol Med. 1975 Mar;148(3):688–693. doi: 10.3181/00379727-148-38611. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo P. C., Lee M. H., Kelly R. K. The polypeptides of infectious pancreatic necrosis virus. J Gen Virol. 1974 Mar;22(3):421–423. doi: 10.1099/0022-1317-22-3-421. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Malsberger R. G., Cerini C. P. Multiplication of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):320–327. doi: 10.1111/j.1749-6632.1965.tb14283.x. [DOI] [PubMed] [Google Scholar]
- Nicholson B. L. Effect of actinomycin D on the multiplication of the infectious pancreatic necrosis virus of trout. Experientia. 1971;27(11):1362–1363. doi: 10.1007/BF02136741. [DOI] [PubMed] [Google Scholar]
- Nicholson B. L. Macromolecule synthesis in RTG-2 cells following infection with infectious pancreatic necrosis (IPN) virus. J Gen Virol. 1971 Nov;13(2):369–372. doi: 10.1099/0022-1317-13-2-369. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Swaney J. B., Vande Woude G. F., Bachrach H. L. Sodium dodecylsulfate-dependent anomalies in gel electrophoresis: alterations in the banding patterns of foot-and-mouth disease virus polypeptides. Anal Biochem. 1974 Apr;58(2):337–346. doi: 10.1016/0003-2697(74)90201-2. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]