Abstract
Repeatability of in vivo measurement of multi-component T2* relaxation in articular cartialges in human knee is important to clinical use. This study evaluated the repeatability of two-component T2* relaxation on seven healthy human subjects. The left knee was scanned once a day in three consecutive days, on a clinical 3T MRI scanner with 8-channel knee coil and ultrashort echo time (UTE) pulse sequence at eleven echo times (TE=0.6-40ms). The intra- and inter-subject repeatability was evaluted via coefficient of variation (CV=SD/mean) in four typical cartilage regions: patellar, anterior articular, femoral and tibial regions. It was found that the intra-subject repeatability was good, with CV<10% for the short- and long-T2* relaxation time in the layered regions in the four cartilages (with one exception) and CV<13% for the component intensity fraction (with two exceptions). The inter-subject repeatability was also good, with CV~8% (range 1-15 %) for the short- and long-T2* relaxation time and CV~10% (range 2-20 %) for the component intensity fraction. The long-T2* component showed significantly better repeatability (CV~8%) than the short-T2* component (CV~12%) (p<0.005). These CV values suggest that in vivo measurement of two-component T2* relaxation in the knee cartilages is repeatable on clinical scanner at 3T, with a signal-to-noise ratio of 90.
Keywords: Repeatability, T2* relaxation, multiple component, knee cartilage, UTE imaging
INTRODUCTION
Proton (1H) spin-spin relaxation, or T2 relaxation, has multiple-component exponential decay in articular cartilages (1-5), reflecting difference in biochemical and biophysical microenvironment surrounding spins in the compartments of extracellular matrix in cartilage such as water/fluid, proteoglycans and collagen fibers. Quantification of compartmental T2 relaxation properties (e.g., relaxation time and component intensity) may render opportunities to non-invasively assess micro damages in a cartilage. Repeatability of the quantification is therefore an important issue to be addressed in the course of advancing this technology.
Four components of T2 relaxation have been identified by nuclear magnetic resonance (NMR) relaxation experiments on bovine knee cartilage explants (1): ultra short T2 (0.02-0.03 ms) from collagen or proteoglycan (PG) macromolecules, very short T2 (~1 ms) from fragmented PG molecules, short T2 (~4 ms) from water molecules trapped within collagen fibrils, and long T2 (~22 ms) from free water molecules. Notably, these T2 components are distinguished from each other in relaxation time and as a result, have the potential to be imaging marker for evaluating alterations of corresponding compartments in the extracellular matrix.
The four components of T2 relaxation described above are detectable on clinical MRI scanners via effective T2 relaxation (or T2* relaxation) by using ultrashort echo time (UTE) pulse sequences. With an UTE of 0.008 ms (6, 7), for instance, all of the four components are detectable. Selective observations are also possible, by properly choosing echo time (e.g., TE=0.6ms) for specifically detecting the short (trapped water) and long (free water) components (4), or by saturating the long-component (free water) for detecting the short and ultrashort components (trapped water, fragmented PG molecules and collagen/PG macromolecules) (8-10).
From clinical perspective, the short T2 relaxation from trapped water molecules is of most interest due to its close relevance to the organization of collagen fibers. Disorganization of collagen fibers, which is an early sign of cartilage degeneration (11-14), results in release of trapped water molecules and the corresponding T2 relaxation time decreases as only strongly-bounded water molecules remain trapped within the disrupted collagen fibrils. Therefore, the T2 relaxation from trapped water molecules has potential to be an imaging marker for detecting collagen fiber disruption. To measure T2 relaxation of the trapped water molecules, a mild ultrashort echo time of ~0.6 ms and two-component relaxation model are appropriate. The 0.6ms-TE helps eliminate signals from very short components related to collagen, PG and fragmented PG macromolecules. The two-component model is needed to separate short (trapped water) from long (free water) component via bi-exponential curve fitting. As spin echo is not available in UTE imaging on clinical MRI scanners, effective T2 (i.e., T2*) relaxations are instead measured.
Curve fitting to the two-component model of T2* relaxation is not trivial, nor is just a nonlinear bi-exponential fitting (3, 5, 15). The major challenge to the fitting is the robustness of fitting process in the presence of mismatch of the model with observed data because T2* relaxation sometimes shows substantial departure from exponential decay. It might be a Gaussian decay due to dephasing of spins caused by inhomogeneity of local magnetic field or a delayed exponential decay due to nonlinear interaction between spins and surrounding tissues (4). Consequently, a pre-processing is necessary to identify large model mismatches and exclude them from fitting process. In addition, a trade-off of fitting accuracy for minimum number of relaxation components is needed in case of small model mismatch. A semi-auto approach, reported in our previous work (4), is therefore a practical choice for two-component curve fitting.
Measurements of T2* relaxation time and component intensity of the short- and long-components are potentially affected by several factors that may be sources of variability such as subject positioning in the magnet, B0 field shimming, slice location, segmentation of region of interest (ROI), error in curve fitting, signal-to-noise ratio (SNR), and the magic angle effect. These potential sources of variability make the repeatability of T2* relaxation measurement very challenging. Previous studies in this area have addressed variability from SNR and magic angle effects (3-5, 15-17; 7, 18-20). The other concerns will be collectively addressed in the following repeatability assessment.
In this study the repeatability of in vivo measurement of two-component T2* relaxations in articular cartilages in human knee was investigated on a clinical MRI scanner at 3T. Measurements on individual subjects across three consecutive days were designed to evaluate the repeatability, and the averaged repeatability over subjects was used to report fluctuation of the repeatability across subjects.
METHODS AND MATERIALS
Human subjects
Eleven human subjects with asymptomatic (healthy) knees were recruited. The study was approved by the author’s Institutional Review Board (IRB) and the consent forms were signed by the study subjects. Left knees were investigated in this study, without intentional preference to this selection. Four subjects were excluded from the evaluation of repeatability (3 for visible motion across echo times in at least one session of the three-session repeat scans and 1 for incomplete three-session scans). Seven subjects (age 28.7±5.0 yr, male/female 5/2) who completed all the three-session scans without visible motion were entered into data analysis for evaluating repeatability.
Experiment design
For this repeatability study, it is critical to keep the same cartilage regions measured under the same experimental conditions. To that end, three session scans were performed on each of the subjects on three consecutive days, same time window on each day (6:30-8:00 pm). The subjects were asked to keep their daily physical activities as consistent as possible across this three-day study period. All the subject/coil set-ups and MRI scans were carried out by the same person and the low periphery of the patella served as positioning marker for all the subjects across the three session scans. Data processing for multi-component T2* fitting was performed by one person, including selection of the slices and segmentation of the region of interest (ROIs). All the slices and ROIs were kept as consistent as possible across sessions for an individual subject and across subjects by using anatomical markers such as femoral/tibal bones, cartilages, vessels, and the interface between cartilage and bone or between vessel and muscle.
MRI scans
The three-session MRI scans were conducted on a 3T clinical MRI scanner (Magnetom Trio Tim, Siemens Medical Solutions, Erlangen, Germany) with an 8-channel knee coil (Invivo Inc., Gainesville, FL). The MRI system has maximum gradient amplitude of 40 mT/m and maximum slew rate of 170 mT/m/ms. The B0-field shimming was implemented manually to achieve minimum linewidth of free induction decay (FID) (<60 Hz) during preparation scan through the adjustment task card on the scanner. Isocenter positioning was implemented on each knee joint. Motion of the knee joint during the scan was minimized by foam padding. The pulse sequence of acquisition-weighted stack-of-spirals (AWSOS) was employed for data acquisition (21), with Monte Carlo simulation optimized, non-uniformly spacing, eleven echo times: TE=0.6, 1, 2, 3, 4, 5, 7, 10, 20, 30, and 40 ms (4). Other acquisition parameters were: sinc RF pulse (0.8ms duration and 1.5 cycles), fat saturation, TR/θ=80ms/30°, slices=60 at thickness 2 mm, FOV=140mm, matrix size=256, nominal resolution =0.55mm, in-plane spirals=24, spiral readout time Ts=11.52ms, averages=1, and total acquisition time TA =1 min 55 sec for each TE in a fashion of one RF excitation for single TE acquisition.
Motion detection and correction
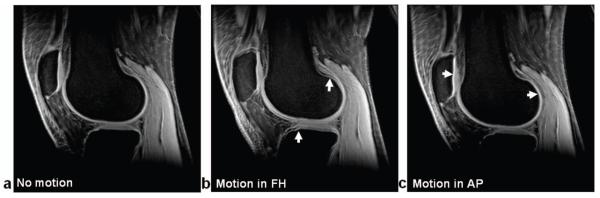
Motion of the knee in the sequential TEs during a session of MRI scans was visually detected both by playing images along TE continuously and by examining image blurring on the maximum intensity projection (MIP) image across TEs. Image jump along TE and/or image blurring on the MIP are indicative of motion of the knee (Fig. 1). Quantification of motion in in-plane (two dimensional) was implemented in cartilage regions of hyper-intensity. The interface between patellar cartilage and bone was used to measure motion displacement in anterior-posterior (AP) direction while the interface between tibial cartilage and bone, or between femoral cartilage and bone, was used in foot-head (FH) direction, for sagittal slices. Cross correlation of image intensity profiles between TEs gives motion displacement at a pre-set accuracy of 0.1 pixel sizes. Motion correction was implemented in the k-space by changing the phase of data points using the measured displacements (22).
Fig. 1.
MIP images across echo times (TEs) of a study subject: (a) without motion, (b) with motion in Foot-Head direction (arrows), and (c) with motion in Anterior-Posterior direction (arrows).
Selection of ROIs
Four typical locations were selected in the cartilages on sagittal slice in lateral side of the knee: patellar cartilage (PC), anterior articular cartilage (AAC), femoral cartilage (FC) and tibial cartilage (TC) (Fig. 2). The lateral side was chosen without any intention against medial side because healthy subjects were studied. Superficial and deep layers (divided through the center of entire cartilage thickness) were segmented manually, leading to eight regions of interest (ROIs) for each subject (Fig. 2a-d). As T2* relaxation significantly changes with depth inside cartilage, cares were taken for the division between the superficial and deep layers by 4× magnifying images. Full thickness regions for the four locations were achieved by combining superficial and deep regions. At the image resolution 0.55mm used in this study, there were 9, 9, 6, and 8 pixels along thickness of the cartilage in PC, AC, FC and TC regions of interest respectively, with 1 or 2 pixels in difference across subjects. The pixels at the cartilage surface or bottom were excluded from T2* evaluation due to their high risk of partial volume effect. Total number of pixels in a full-thickness ROI was 57±16, 40±12, 62±17, or 47±17 (mean±SD) for the PC, AAC, FC and TC regions of interest among the subjects studied, respectively.
Fig. 2.
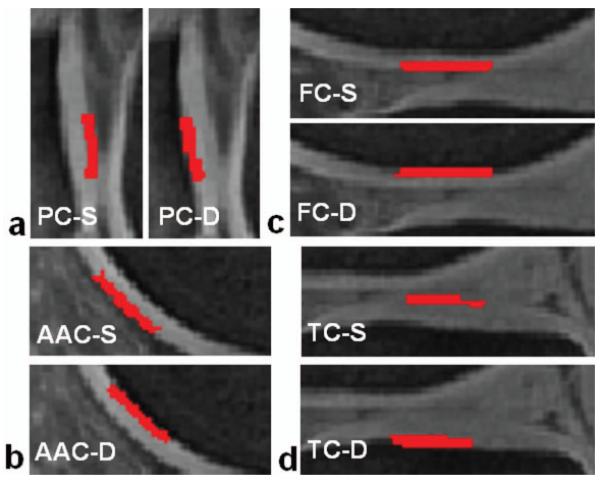
Regions of interest (ROIs) for repeatability evaluation at superficial (S) and deep (D) layers in each panel (red regions): (a) patellar cartilage (PC), (b) anterior articular cartilage (AAC), (c) femoral cartilage (FC), and (d) tibial cartilage (TC).
Model of two-component T2* relaxations and bi-exponential fitting
A model of two T2* components was formulated in Eq. [1], with measured image intensity s(TE), unknown component intensity and relaxation time constant (A21, ) for trapped and (A22, ) for free water molecules, and background noise n(TE). As a very limited number of TEs is available in clinical setting (only 11 TEs in this study), statistical properties (e.g., mean and standard deviation) of the background noise along the TE direction at a voxel are most-likely biased from those for whole image background noise. Therefore, it is appreciated to consider the noise in the Eq. [1] as an unknown.
| Eq. [1] |
| Eq. [2] |
| Eq. (3) |
A semi-auto algorithm was employed to perform the curve fitting to the model described in Eq. [1] on a voxel-by-voxel basis (4). First, a pre-processing was applied to the measured decay at a voxel to identify non-exponential decay and exclude it from the fitting process. Then, the non-negative least squares (NNLS) algorithm was applied to the exponential decays, with an initial guess of nine T2* values [i.e., T2*initial = (0.5, 2, 5, 10, 15, 25, 40, 60, 90) ms] covering possible relaxation times of the short- and long-T2* components. The initial guess was the same for all the voxels investigated in this study. The noise term in Eq. [1] was incorporated into the NNLS algorithm by introducing an extra T2* relaxation of very large time constant (e.g., T2*noise = 350 ms) but of variable component intensity to be determined [i. e., ]. Modify (i.e., increase or decrease) the T2* values until only one or two components have non-zero component intensities. To eliminate scaling effect of radiofrequency (RF) excitation and receiver coil sensitivity on image intensity across subjects and across scan sessions, intensity fraction of a T2* component, such as a21 or a22 defined in Eqs. [2, 3], was reported in this study, instead of absolute component intensity A21 or A22. The scaling of image intensity across TEs in a single scan session for an individual subject was maintained the same by using the same coil voltage for RF excitation and the same coil FFT factors for signal receive (the scaling process may differ on different MRI systems).
For comparison, a single-component model was also used and mono-exponential fitting to decay curve (without constant term) was implemented. The corresponding fitting outcome was reported in this study as single-component T2* value at a voxel.
Repeatability standard deviation
For each subject, the mean and standard deviation (SD) of T2* relaxation time and component intensity across the three-session scans were calculated respectively, and the coefficient of variation (CV=SD/Mean) was reported as repeatability for individual subjects (or intra-subject repeatability). The repeatability across subjects, or inter-subject repeatability, was estimated by averaging the intra-subject repeatability over the N subjects. Both the mean coefficient of variation [MEAN-CV=Σ(CVi)/N)] and root-mean-squares coefficient of variation [] were evaluated for reporting inter-subject repeatability.
Statistical analysis
Statistical significance (p-value) of the measurements attained in this study was evaluated using the paired/unpaired Student’s t-test with one-/two-tailed distribution depending on the type of comparison. A standard threshold (p=0.05) was chosen to define statistical significance in this study. The p-values presented below were calculated using the software of Microsoft Excel (Microsoft, 2002).
RESULTS
In vivo SNR
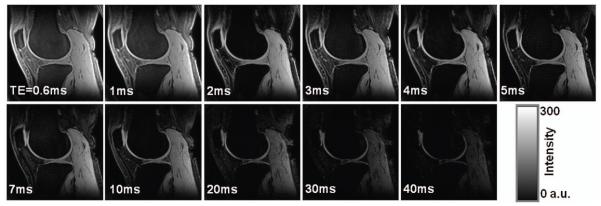
As expected, signal intensity in the cartilages decreased with TE increasing and the signal decreasing was faster in the deep layer of cartilages than in the superficial (Fig. 3). The signal-to-noise ratio (SNR) measured on the patellar cartilage by taking the ratio of the averaged signal intensity in a cartilage ROI to the standard deviation (SD) of noise in a noise-only background region, was 87, 92, 81, 71, 66, 59, 47, 43, 29, 23 and 16 at TE=0.6-40ms, respectively. These values demonstrates that the in vivo SNR was high enough for the bi-exponential fitting used in this study, based on the knowledge acquired from our previous studies by Monte Carlo simulations (4).
Fig. 3.
Images of a subject across the 11 echo times (TEs), displayed at the same window/level. Signal intensity in the deep layer of cartilages decreased faster with TE increasing than in the superficial layers, due to faster T2* relaxation. SNR on the patellar cartilage is 87, 92, 81, 71, 66, 59, 47, 43, 29, 23, and 16 for TE=0.6-40ms, respectively. (3T, FOV=140mm, matrix=256, thickness=2mm, TR=80ms, θ=30°)
T2* decays at individual voxels
It was observed in this study that in vivo T2* decay at an individual voxel was not always exponential but had four most-popular types of mono-, bi-, Gausian and other non-exponential decays as demonstrated in Figure 4. The delay time for the non-exponential decay was measured by the time when normal exponential decay starts. The T2* time in the Gaussian decay was estimated via the term of exp[−(TE/T2*)2]. The mono- and bi-exponential decays in Figure 4 are distinct from each other in curve shape. This difference is further demonstrated in Figure 5. The measured signal intensities are [248.8, 249.9, 197.1, 163.6, 148.7, 117.1, 91.5, 74.8, 49.6, 33.4, 17.9] (arbitrary unit) at TE = [0.6, 1, 2, 3, 4, 5, 7, 10, 20, 30, 40] ms, respectively. Two curve fittings (bi- and mono-exponentials) on the measured T2* decay suggests that the bi-exponential fitting is better than the mono-exponential fitting to this particular case, with residual fitting error 4% vs. 20% relative to the measured decay (Fig. 5a). The mono-exponential model used here has a constant term of 8.2 (arbitrary unit), which is the averaged mean of background noise measured on the magnitude images over the eleven TEs. The background noise was measured in a noise-only region of 570 pixels outside of the knee joint for each of the TE images. The mean and standard deviation (SD) of the background noise for individual TEs were shown in Figure 5.
Fig. 4.
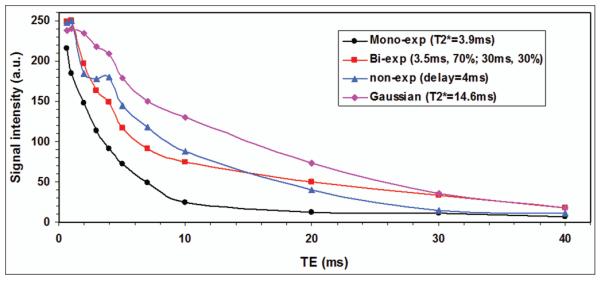
Representative signal decay curves of T2* relaxation at individual voxels in the knee cartilages of the study subjects, with mono-, bi- or non-exponential decay, or Gaussian decay. The delay time (4ms) for the non-exponential decay was measured by the time when the normal exponential decay starts. The T2* time in the Gaussian decay was estimated via the term of exp[−(TE/T2*)2].
Fig. 5.
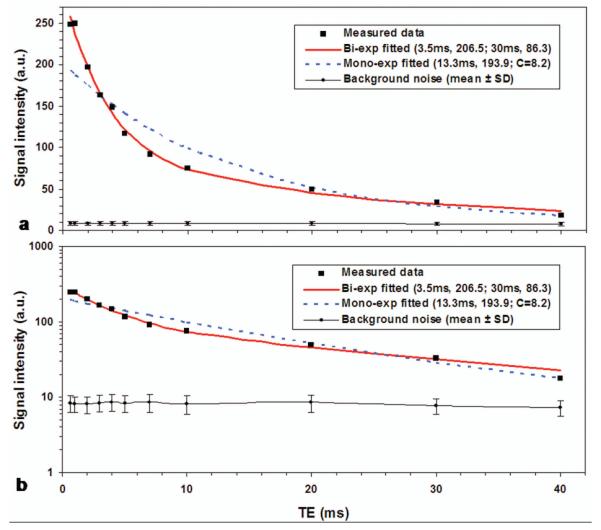
Measured T2* decay at a voxel in cartilage of a study subject and the curve fittings to it; a) in decimal scale for showing fitting goodness and b) in logarithmic scale for showing existence of more than one exponential terms (i.e., departing from a straight line). The bi-exponential model produced better fitting to the measured data than the mono-exponential model, with fitting error 4% vs. 20%. Note: the mono-exponential model has a constant term of 8.2 (a.u.), which is the averaged mean of background noise (as shown in a & b) measured on the magnitude images over all the eleven TEs.
The departure from a straight line of the measured data points in logarithmic scale (Fig. 5b) suggests that there be more than one component in the measured T2* decay and that there be a need of two-component model and associated bi-exponential fitting for T2* decays in cartilages in the knee. On the other hand, the two-component model is still not general enough to include the Gaussian and other non-exponential T2* decays as shown in Fig. 4. Those non-exponential decays have large mismatches between the model and measured T2* decays. This supports our choice of the semi-auto algorithm that excluded the Gaussian and other non-exponential decays from the fitting process of T2* relaxation in this study. If a T2* map for short- or long-component is to be constructed, a value of −1.0 (or other negative numbers) is assigned to label the pixels of non-exponential decay. One should not perform quantitative evaluations of T2* time on these pixels.
Measurements of T2* relaxation properties
The measurements of short component (T2*S, a21) and long component (T2*L, a22), as well as single component (T2*single) (that was obtained by the curve fitting with single exponential component), are listed in Table 1. The patellar and anterior articular cartilages have short-T2* time of 3.3-3.7 ms and the femoral/tibial cartilages have 2.8-2.9 ms, but the difference is not statistically significant (p=0.07). However, they have component intensity fraction (31-48 %) significantly smaller than the femoral/tibial cartilages (68-89 %) (p<0.03). The patellar, femoral and tibial cartilages have similar long-T2* time (17-24 ms) while the anterior articular cartilage has distinctly longer one (41 ms). Single-component fitting shows similar pattern for the four cartilages (12-14 ms for PC, FC/TC and 24 ms for AAC) as the long component illustrated. The variation of relaxation time from the femoral/tibial ROI (in which the orientation angle of radial collagen fibers to the main magnetic field was ~0°) to the anterior articular ROI (the orientation angle was ~45°) is 18% for the short component but 70% for both long and single components. This suggests that magic angle effect on relaxation time for the short-component may be about four times smaller than for the long- or single-component (18% vs. 70%).
Table 1.
Intra-subject repeatability*.
| (T2*)short | a2 | (T2*)long | a22 | (T2*)single | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| ROI | Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | Mean | i SD | CV |
| (ms) | (ms) | (%) | (%) | (ms) | (ms) | (%) | (%) | (ms) | (ms) | ||||||
| Patellar cartilage (PC) | |||||||||||||||
| Superficial | 5.02 | 0.08 | 1.6% | 34.3 | 3.2 | 9.3% | 35.8 | 2.4 | 6.7% | 81.5 | 0.1 | 0.2% | 27.0 | 1.1 | 4.0% |
| Deep | 3.79 | 0.16 | 4.2% | 66.4 | 7.4 | 11.1% | 22.1 | 1.3 | 5.9% | 42.5 | 5.3 | 12.5% | 7.8 | 0.9 | 11.5% |
| Full | 3.71 | 0.21 | 5.7% | 48.4 | 7.5 | 15.5% | 21.9 | 2.1 | 9.6% | 53.3 | 7.8 | 14.6% | 14.7 | 0.5 | 3.4% |
| Anterior articular cartilage (AAC) | |||||||||||||||
| Superficial | 3.38 | 0.25 | 7.4% | 36.3 | 0.6 | 1.7% | 44.1 | 4.1 | 9.3% | 68.9 | 2.0 | 2.9% | 18.9 | 3.0 | 15.9% |
| Deep | 1.65 | 0.06 | 3.6% | 16.6 | 0.1 | 0.6% | 32.2 | 0.3 | 0.9% | 92.1 | 1.6 | 1.7% | 29.8 | 0.8 | 2.7% |
| Full | 3.29 | 0.21 | 6.4% | 31.2 | 4.3 | 13.8% | 40.7 | 1.7 | 4.2% | 78.4 | 3.5 | 4.5% | 23.8 | 0.3 | 1.3% |
| Femoral cartilage (FC) | |||||||||||||||
| Superficial | 3.90 | 0.14 | 3.6% | 87.0 | 18.4 | 21.1% | 23.5 | 5.5 | 23.4% | 80.6 | 8.9 | 11.0% | 17.2 | 0.5 | 2.9% |
| Deep | 2.00 | 0.15 | 7.5% | 99.4 | 0.8 | 0.8% | - | - | - | - | - | - | 2.57 | 0.52 | 20.2% |
| Full | 2.84 | 0.34 | 12.0% | 89.2 | 15.2 | 17.0% | 23.5 | 5.5 | 23.4% | 80.6 | 8.9 | 11.0% | 12.0 | 1.9 | 15.8% |
| Tibial cartilage (TC) | |||||||||||||||
| Superficial | 5.84 | 0.01 | 0.2% | 34.9 | 7.7 | 22.1% | 19.7 | 0.1 | 0.5% | 91.0 | 0.6 | 0.7% | 19.9 | 0.5 | 2.5% |
| Deep | 2.24 | 0.06 | 2.7% | 77.3 | 7.8 | 10.1% | 12.6 | 0.2 | 1.6% | 65.8 | 3.5 | 5.3% | 9.4 | 0.7 | 7.4% |
| Full | 2.94 | 0.46 | 15.6% | 68.8 | 4.2 | 6.1% | 17.1 | 0.7 | 4.1% | 82.1 | 0.4 | 0.5% | 15.5 | 0.2 | 1.3% |
The component intensity fractions a21 and a22 do not sum up to 100% because they are not from individual voxels. Instead, they are from a ROI and averaged over the three sessions of MRI scan. CV=mean/SD.
The deep layers in the cartilages, as expected, have component intensity fractions (66-99 %) of the short component clearly higher than the superficial layers (34-87 %) (p<0.05), except for AAC where the values are reversed.
Intra-subject repeatability
The intra-subject repeatability (i.e., repeating measurements on the same subject) is listed in Table 1 and described by coefficient of variation (CV). At full thickness, the short-component T2* relaxation time has good repeatability (5.7-15.6 %) in the four cartilages studied, with better repeatability in the patellar and anterior articular cartilages (5.7 and 6.4 %) than in the femoral and tibial cartilages (12.0 and 15.6 %). But this difference is not statistically significant (p=0.06). In the anterior articular and tibial cartilages, however, the long-component T2* relaxation time have repeatability of 4.1 and 4.2 %, different from 9.6 and 23.4 % in the patellar and femoral cartilages. This difference is however not statistically significant (p=0.17). The single-component T2* relaxation time shows great repeatability (1.3-3.4%) in the four cartilages studied, except the femoral cartilage (15.8%). The component intensity fraction has similar repeatability to the relaxation time, with 6.1-17.0 % for the short-T2* and 0.5-14.6 % for the long-T2* relaxations in the four cartilages (the difference is not statistically significant, p=0.26). Notably, the tibial cartilage shows very high repeatability of the intensity fraction for both short- and long-T2* relaxations (0.5 and 6.0 %).
At the deep layers, both short- and long-T2* relaxations have good repeatability, with CV < 8% for relaxation time and CV<13% for component intensity fraction, compared with the single-T2* relaxation time, with CV=2.7-20.2 %. At the superficial layers, the short- and long-T2* relaxations share again high repeatability of both relaxation time (CV<10%) and intensity fraction (CV<11%) in the four cartilages, except the long-T2* relaxation time in the femoral cartilage (23.4%) and the short-T2* intensity fraction in femoral/tibial cartilages (21.1-22.1 %).
Overall, the measurements of short- and long-T2* relaxation time have high repeatability (CV<10%) in the layered regions in all the four cartilages studied, with one exception of the long-T2* relaxation in the superficial layer of femoral cartilage (23.4%). The measurements of component intensity fractions also illustrate high repeatability (CV<13%), with two exceptions of short-T2* relaxation in the superficial regions in femoral and tibial cartilages.
Inter-subject repeatability
The inter-subject repeatability (i.e., repeatability on different subjects) is reported with both mean CV (or CV for short) and root-mean-squares (RMS) CV (Figs. 6,7). The RMS-CV value is slightly, but always (p<0.000002), larger than the CV value, showing small fluctuation of CV across subjects.
Fig. 6.
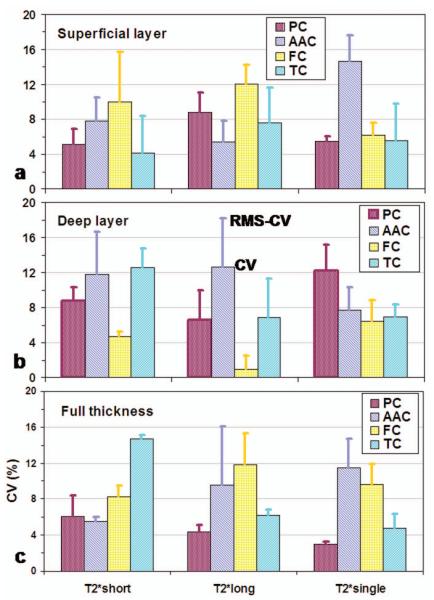
Repeatability of T2* time across subjects for the superficial (a), deep (b) and full-thickness (c) layers, described by averaged coefficient of variation (CV) across subjects (solid bar), or by the root-mean-square (RMS) CV across subjects (error bar).
Fig. 7.
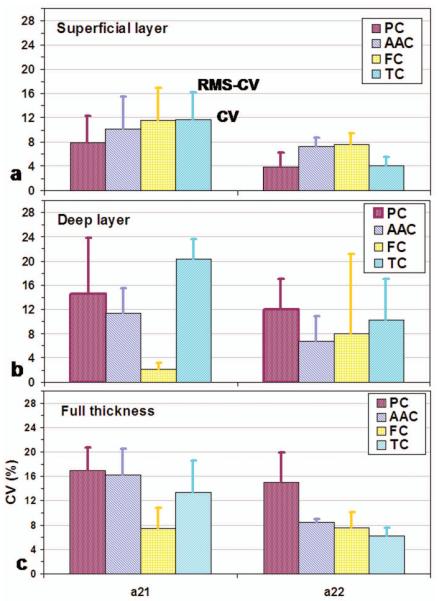
Repeatability of T2* component intensity fraction (a21 and a22) across subjects.
For the relaxation time (Fig. 6), the repeatability is very good, with CV at ~8% (in a range of 1-15 %) among the short-, long- and single-T2* relaxations in all the four cartilages, either layered regions or full thickness. For component intensity fraction (Fig. 7), the repeatability is slightly worse than that of the relaxation time (p<0.04), with CV at ~10% (in a range of 2-20 %) for the short- and long-T2* relaxations. In general, the long-T2* component shows significantly better repeatability (CV~8%) than the short-T2* component (CV~12%) (p<0.005). In the deep layer of tibial cartilage, short-T2* has the lowest repeatability (20%).
DISCUSSION
This study has demonstrated good repeatability of in vivo measurement of the T2* relaxation properties (time constant and component intensity) for short- and long-components. This achievement was largely based on the high signal-to-noise ratio and small motion artifacts in the images acquired in this study by means of 8-channel coil and motion control. The semi-auto algorithm for the bi-exponential fitting was another major contributor to the high repeatability as it excluded non-exponential T2* decays from the fitting process. The non-exponential T2* decays had large mismatches with the two-component exponential model and thus had potential to produce large uncertainty of the fitting outcome.
The relaxation time of the short-, long- and single-components at full thickness has higher intra-subject repeatability in the patellar, anterior articular, and tibial cartilage regions than in the femoral cartilage region (Table 1). This was due mainly to lower SNR in the femoral region. Thin thickness of femoral cartilage was also responsible for the low repeatability (23.4%) of long-T2* relaxation time in the superficial region due to rapid change of T2* relaxation from the superficial to deep layer.
The intra-subject repeatability for the full thickness regions of interest was not the one that just was averaged over the layered regions of interest (Table 1). This was because large difference in the mean and deviation between the layered and full thickness regions of interest.
Magic angle effect on the relaxation time was four times smaller for the short-T2* component than for the long-T2* component as shown in the anterior articular and femoral/tibial regions (Table 1). This might be due to the free water more sensitive to collagen fiber orientation than trapped water as a fast exchange exists between free water molecules and water molecules in the water sheath on the surface of collagen molecule (19, 20, 23). That The magic angle has less effect on short-T2 value than on long-T2 value was already demonstrated in bovine tendon explants by Fullerton et at in 1985 (24), where the short-T2 value increased from bottom to peak by a factor of ~2 but the long-T2 value by a factor of ~4. This phenomenon might be a good sign for short-T2* relaxation to be an imaging marker that is less sensitive to magic angle effect.
The inter-subject repeatability of relaxation time and component intensity was shown in general better for the long-T2* relaxation (8%) than for the short-T2* relaxation (12%) due in part to larger potential differences among individuals.
Slice location was a source of measurement error that might degrade repeatability. It was not possible to have slices exactly at the same cartilage location between the measurements at different days or different subjects. A half slice thickness might be shifted at most among the repeat measurements and that certainly introduced errors to the T2* measurements.
SNR requirement for curve fitting depends on difference in T2* time between two components to be resolved. For the two distinct T2* components of our interest (~4ms from the trapped and ~22ms from the free water), SNR at ~90, which is achievable on clinical scanners at 3T as shown in Fig. 3 in this work, is high enough for the bi-exponential fitting as demonstrated in our Monte Carlo simulations (4). The works in References 3,5,16 are also supportive of this argument. However, it is worth to mention that a SNR of 90 is good for bi-exponential fitting at the level of a region of interest, instead of at the level of a single pixel. There were 30 or more pixels in a ROI used in this study. This means that more than five times high SNR (i.e., ~500) was actually used in this study for reporting repeatability of T2* values at a ROI. This SNR value is within the range reported by Graham et al (15). To repeat T2* values of two components at the level of a single pixel, a SNR of ~500 is surely needed. One way to achieve it in clinical setting is to acquire more TE points, such as >64 of TEs. This is very challenging to in vivo studies on human, but it is possible technically.
Magic angle effect has been known to introduce extra variation of T2 (and T2*) relaxation time due to collagen fiber orientation relative to main magnetic field B0 (7, 18-20). Although this distractive effect has large impact on ex vivo measurements of T2 relaxation time on cartilage explants due to unlimited positioning freedom of the explants (7, 18), it has small impact on in vivo measurements on human due to limited positioning freedom of the knee joint inside a magnet. Spatial modulation of the magic angle effect on in vivo T2 time measurement on knee cartilages is regional and removable (i.e., correctable via post data processing), and thus should not have substantial impact on the evaluation of local disruptions of collagen fibers. In addition, it was found in this study that magic angle effect on relaxation time of the short-T2* component is much smaller than for the long-T2* component (18% vs. 70%). This might be due to the short-T2* relaxation originating from water molecules that are already trapped within collagen fibers and thus have less preference of collagen fiber orientation to their movements than free water molecules that produce the long-T2* component (19, 20).
The total scan time (22 min) for the eleven TEs used in this study was acceptable to the 11 subjects studied. In a total of 32 separate scans implemented in this study, scans of visible motion artifacts were at a small rate of 12.5% (or 4/32). However, this rate can be further reduced for the sake of clinical use by reducing total scan time. It is our expectation that, by using multi-TE acquisitions at one excitation, 64-TE acquisitions in the same range of TE=0.6-40ms as used in this study would be completed in eight excitations at a total scan time of ~16 min, without a compromise with image quality. Furthermore, the increased number of TE points would help increase robustness of the bi-exponential fitting and eventually increase repeatability of the measurements of two-component T2* relaxations.
CONCLUSIONS
This study has demonstrated that in vivo measurement of two-component T2* relaxation in the cartilages in healthy human knee joint was repeatable at the level of a region of interest, either across the measurements on a single subject (<10% and <13% in relaxation time and component intensity fraction respectively, for both layered and full-thickness regions) or across the measurements on different subjects (<15% and <20% in relaxation time and component intensity fraction respectively, for both layered and full-thickness regions). The major contributor to these achievements was the high signal-to-noise ratio (SNR ≈ 90) in the cartilage regions available to this study. Three technical components such as the 3T magnet, 8-channel knee coil and UTE sequence were necessary for the available high value of signal-to-noise ratio.
Acknowledgments
This work was supported in part by the Department of Radiology Development Fund, University of Pittsburgh, and by the NIH R01 AR052784.
REFERENCES
- 1.Lattanzio PJ, Marshall KW, Damyanovich AZ, Peemoeller H. Macromolecule and water magnetization exchange modeling in articular cartilage. Magn Reson Med. 2000;44:840–851. doi: 10.1002/1522-2594(200012)44:6<840::aid-mrm4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Mosher TJ, Dardzinski BJ. Cartilage MRI of T2 relaxation time mapping: overview and applications. Semin Musculoskeloskelet Tadiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 3.Reiter DA, Lin PC, Fishbein KW, Spencer RG. Multicomponent T2 relaxation analysis in cartilage. Magn Reson Med. 2009;61:803–809. doi: 10.1002/mrm.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian Y, Williams AA, Chu CR, Boada FE. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med. 2010;64:1426–1431. doi: 10.1002/mrm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du J, Diaz E, Carl M, Bae W, Chung CB, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med. 2012;67:645–649. doi: 10.1002/mrm.23047. [DOI] [PubMed] [Google Scholar]
- 6.Brittain JH, Shankaranarayanan A, Ramanan V, Shimakawa A, Cunningham CH, Hinks S, Francis R, Turner R, Johnson JW, Nayak KS, Tan S, Pauly JM, Bydder GM. Ultra-short TE imaging with single-digit (8μs) TE; Proceedings of the 12th Annual Meeting of ISMRM; Kyoto, Japan. 2004.p. 629. [Google Scholar]
- 7.Du J, Pak BC, Znamirowski R, Statum S, Takahashi A, Chung CB, Bydder GM. Magic angle effect in magnetic resonance imaging of the Achilles tendon and enthesis. Magn Reson Imaging. 2009;27:557–564. doi: 10.1016/j.mri.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Larson PE, Conolly SM, Pauly JM, Nishimura DG. Using adiabatic inversion pulses for long-T2 suppression in ultrashort echo time (UTE) imaging. Magn Reson Med. 2007;58:952–961. doi: 10.1002/mrm.21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmer J, Blume U, Börnert P. Selective 3D ultrashort TE imaging: comparison of “dual-echo” acquisition and magnetization preparation for improving short-T2 contrast. MAGMA. 2007;20:83–92. doi: 10.1007/s10334-007-0070-6. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Bydder M, Takahashi AM, Carl M, Chung CB, Bydder GM. Short T2 contrast with three-dimensional ultrashort echo time imaging. Magn Reson Imaging. 2011;29:470–482. doi: 10.1016/j.mri.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gay S, Miller EJ. Collagen in the physiology and pathology of connective tissue. Gustav Fischer New York Inc.; New York: 1978. p. 110. [Google Scholar]
- 12.Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, Tchetina E, Wu W. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii78–81. doi: 10.1136/ard.61.suppl_2.ii78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmander LS. Markers of altered metabolism in osteoarthritis. J Rheumatol Suppl. 2004;70:28–35. [PubMed] [Google Scholar]
- 14.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:75–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 15.Graham SJ, Stanchev PL, Bronskill MJ. Criteria for analysis of multicomponent tissue T2 relaxation data. Magn Reson Med. 1996;35:370–378. doi: 10.1002/mrm.1910350315. [DOI] [PubMed] [Google Scholar]
- 16.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63:181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 17.Diaz E, Chung CB, Bae WC, Statum S, Znamirowski R, Bydder GM, Du J. Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR Biomed. 2012;25:161–168. doi: 10.1002/nbm.1728. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y. Magic-angle effect in magnetic resonance imaging of cartilage: a review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Bydder M, Rahal A, Fullerton GD, Bydder GM. The magic angle effect: a source of artifact, determinant of image contrast, and technique for imaging. J Magn Reson Imaging. 2007;25:290–300. doi: 10.1002/jmri.20850. [DOI] [PubMed] [Google Scholar]
- 20.Fullerton GD, Rahal A. Collagen structure: The molecular source of the tendon magic angle effect. J Magn Rason Imaging. 2007;25:345–361. doi: 10.1002/jmri.20808. [DOI] [PubMed] [Google Scholar]
- 21.Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution 3D UTE MR imaging. Magn Reson Med. 2008;60:135–145. doi: 10.1002/mrm.21620. [DOI] [PubMed] [Google Scholar]
- 22.Haacke EM, Brown RW, Thompson MR, Venkatesan R. Magnetic resonance imaging-Physical principles and sequence design. John Wiley & Sons, Inc.; New York: 1999. p. 914. [Google Scholar]
- 23.Ikoma K, Kusaka Y, Takamiya H, Eliav U, Navon G, Seo Y. Evaluation of collagen fiber maturation and ordering in regenerating tendons employing H-1 double quantum filtered NMR spectroscopy. J Orthopead Res. 2003;21:149–156. doi: 10.1016/S0736-0266(02)00092-X. [DOI] [PubMed] [Google Scholar]
- 24.Fullerton GD, Cameron IL, Ord VA. Orientation of tendons in the magnetic field and its effect on T2 relaxation times. Radiology. 1985;155:433–435. doi: 10.1148/radiology.155.2.3983395. [DOI] [PubMed] [Google Scholar]