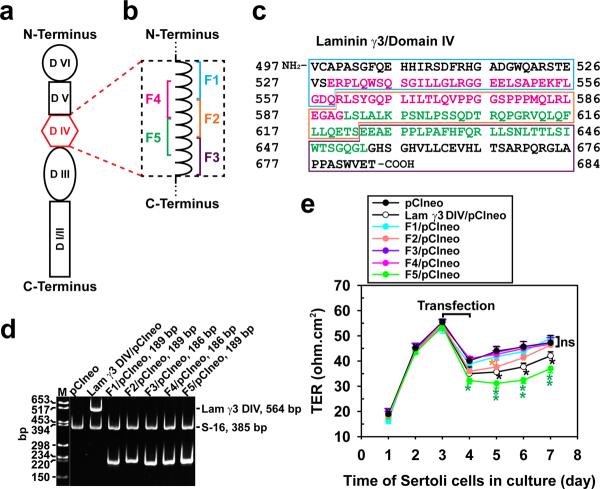
Figure 1. Preparation of laminin-γ3 domain IV cDNA constructs to assess their biological activity.
(a) Schematic drawing illustrating different functional domains of laminin-γ3 chain. Laminin-γ3 domain IV (DIV, in red) was selected based on earlier studies that this domain is biologically active in regulating BTB dynamics,15 and it was further divided into 5 fragments of F1–F5 as shown in (b) to assess their effects on BTB function. (c) cDNA-deduced amino acid sequence of laminin-γ3 DIV (GenBank accession code XM_231139) is shown, illustrating the sequences of F1 (blue boxed area), F2 (orange boxed area), F3 (purple boxed area), F4 (pink colored text) and F5 (green colored text). (d) RT-PCR illustrating the expression of laminin-γ3 DIV and the five different fragments (see a) in Sertoli cells after transfection with corresponding vectors versus pCIneo alone for 3 days. Cells transfected with pCIneo alone served as a negative control since Sertoli cells per se did not express laminin-γ3 chains, which are exclusively expressed by elongating/elongated spermatids.10 S-16 served as an internal PCR control. Identity of the PCR product was confirmed by direct nucleotide sequencing at GeneWiz Inc (South Plainfield, NJ). M, DNA markers; bp, base-pair. (e) Changes in the Sertoli cell TJ-permeability barrier function following transient expression of different fragments of laminin-γ3 DIV versus DIV (positive control) and pCIneo alone (negative control) on day 3 for 24-hr by quantifying TER across the Sertoli cell epithelium in bicameral units (n = 3). This experiment was repeated three times during different batches of Sertoli cells and yielded similar results. *, P<0.05; **, P<0.01.