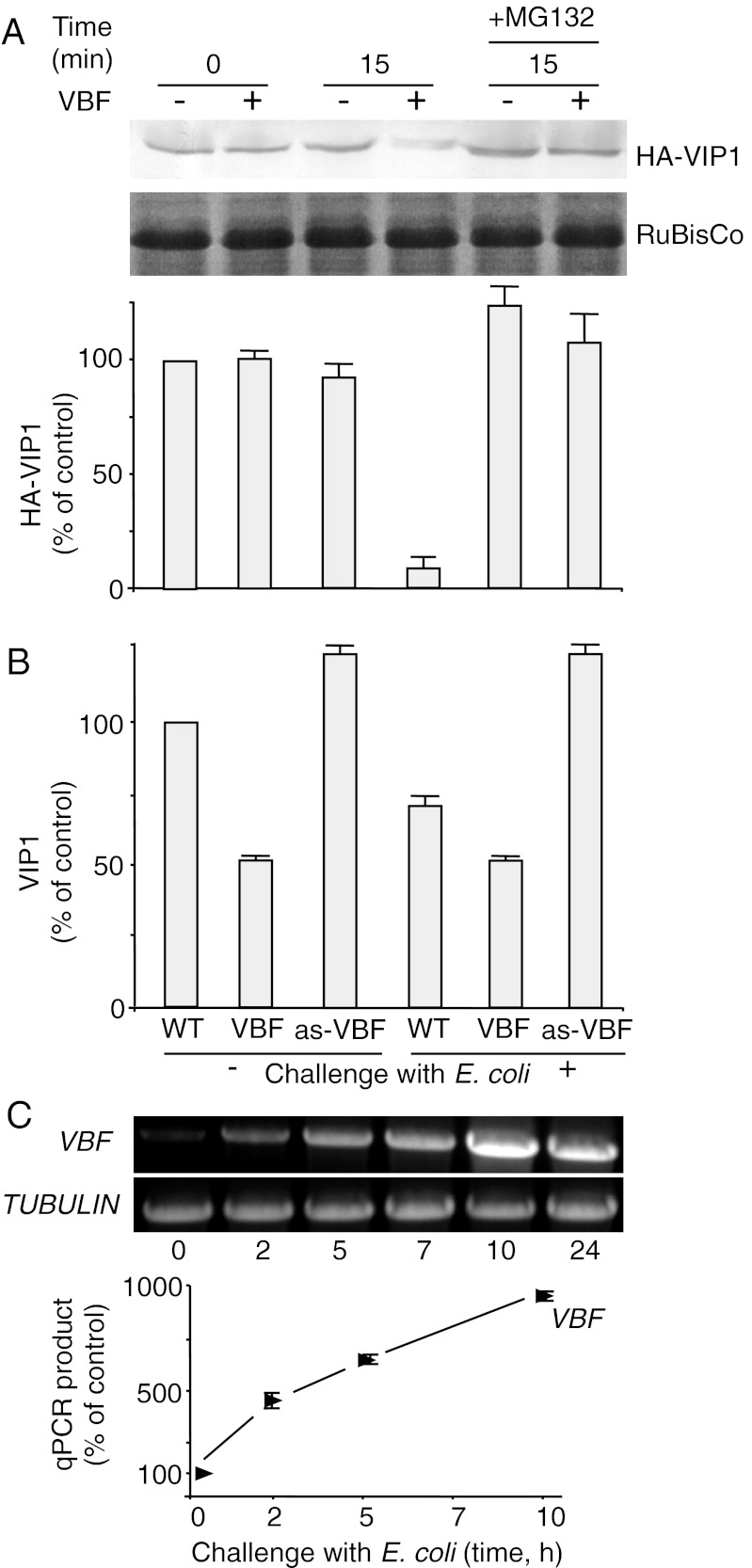
Fig. 1.
VBF promotes proteasomal degradation of VIP1. (A) Western blot analysis of VIP1 degradation in the N. benthamiana cell-free system. HA-VIP1 was expressed alone or coexpressed with VBF in N. benthamiana leaves. The resulting protein extracts were incubated for the indicated time periods and analyzed using anti-HA antibodies. The putative RuBisCo large chain was used as a loading control. The quantified Western blot signal was expressed as percent of the signal obtained in the absence of VBF at the start of the incubation period. The data represent average values of three independent experiments with indicated SDs. (B) Western blot analysis of the endogenous VIP1 content in Arabidopsis plants. VBF expression was induced by challenging the plants for 10 h with E. coli. Protein extracts from WT plants, VBF transgenic plants (VBF), or VBF antisense plants (as-VBF) were analyzed with anti-VIP1 antibody, and the resulting signal was quantified and expressed as percent of the signal obtained in WT, unchallenged plants. The data represent average values of three independent experiments with indicated SDs. (C) RT-PCR (Upper) and RT-qPCR analyses (Lower) of VBF gene expression in WT Arabidopsis plants after challenge with E. coli. Constitutively expressed TUBULIN was used as an internal control.