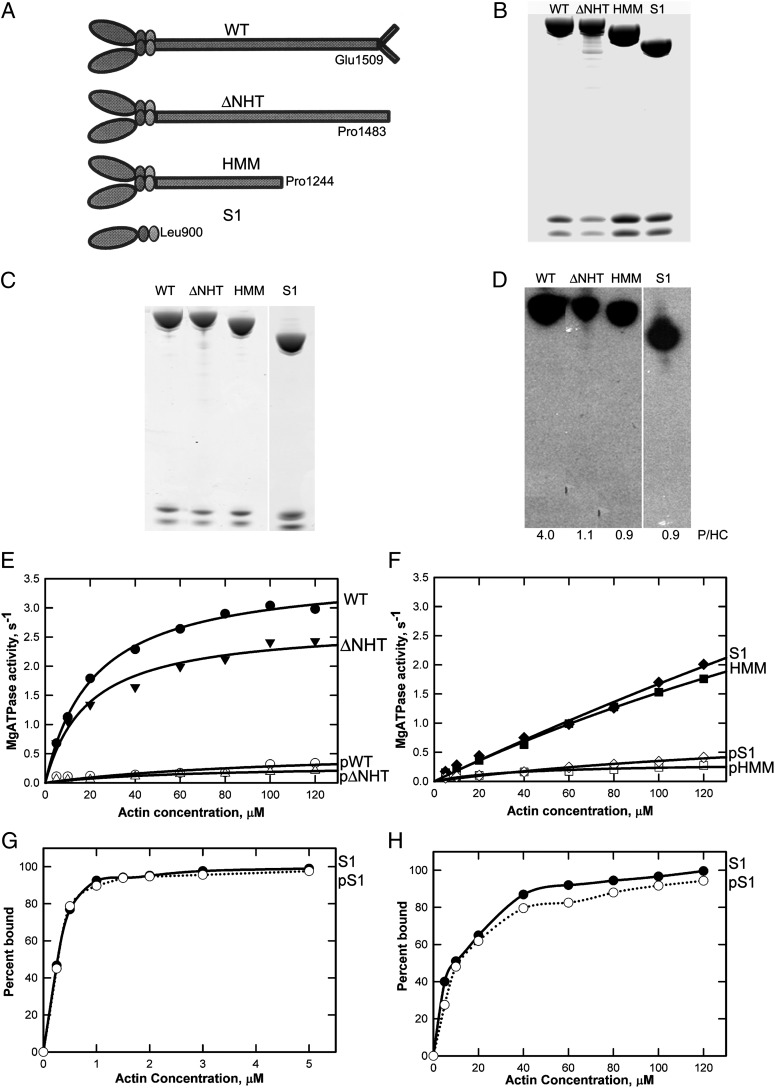
Fig. 1.
Phosphorylation and actin-activated ATPase activity of AMII constructs. (A) Schematic illustration of WT and recombinant myosins ΔNHT, HMM, and S1. (B) Coomassie blue-stained SDS/PAGE of purified recombinant myosins showing the heavy chain and two light chains. Samples were analyzed on a 1.0-mm 10% NuPAGE gel (Life Technologies). (C and D) Coomassie blue-stained SDS/PAGE (C) and autoradiogram (D) of recombinant myosins phosphorylated in vitro by the kinase preparation. The incorporated phosphates per heavy chain (P/HC; average of two to four experiments) is given under the lanes in D. (E and F) Actin dependence of Mg-ATPase activity of unphosphorylated and phosphorylated WT and ΔNHT (E) and HMM and S1 (F). Data are the averages of two experiments. (G and H) Binding of S1 and pS1 to F-actin in 2.5 mM KCl and 3 mM MgCl2 without ATP (G) or in 2.5 mM KCl and 3 mM MgCl2 with 1 mM ATP (H). In the absence of ATP, F-actin was stabilized by phalloidin at a 1:1 molar ratio to actin.