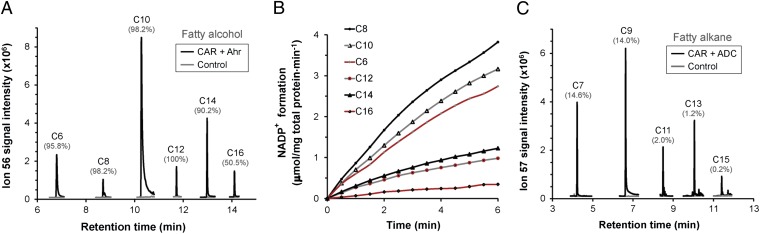
Fig. 4.
Broad-range conversion of fatty acids to fatty alcohols and alkanes. (A) In vitro formation of fatty alcohols. Reactions were carried out in 50 mM Tris⋅HCl buffer containing CARhis (10–200 μg·mL−1), Ahrhis (10 μg·mL−1), 1 mM ATP, 10 mM MgCl2, 1 mM NADPH, and 0.25 mM C6–C16 fatty acids. (B) In vitro rate of conversion of fatty acids to alcohols. Reactions were monitored at 340 nm for 6 min at 30-s intervals. (C) In vitro formation of alkanes. Same as A except Ahrhis was replaced with ADChis. In A and C, the percentage yield of product per substrate over a 4-h period is indicated in brackets above each peak.