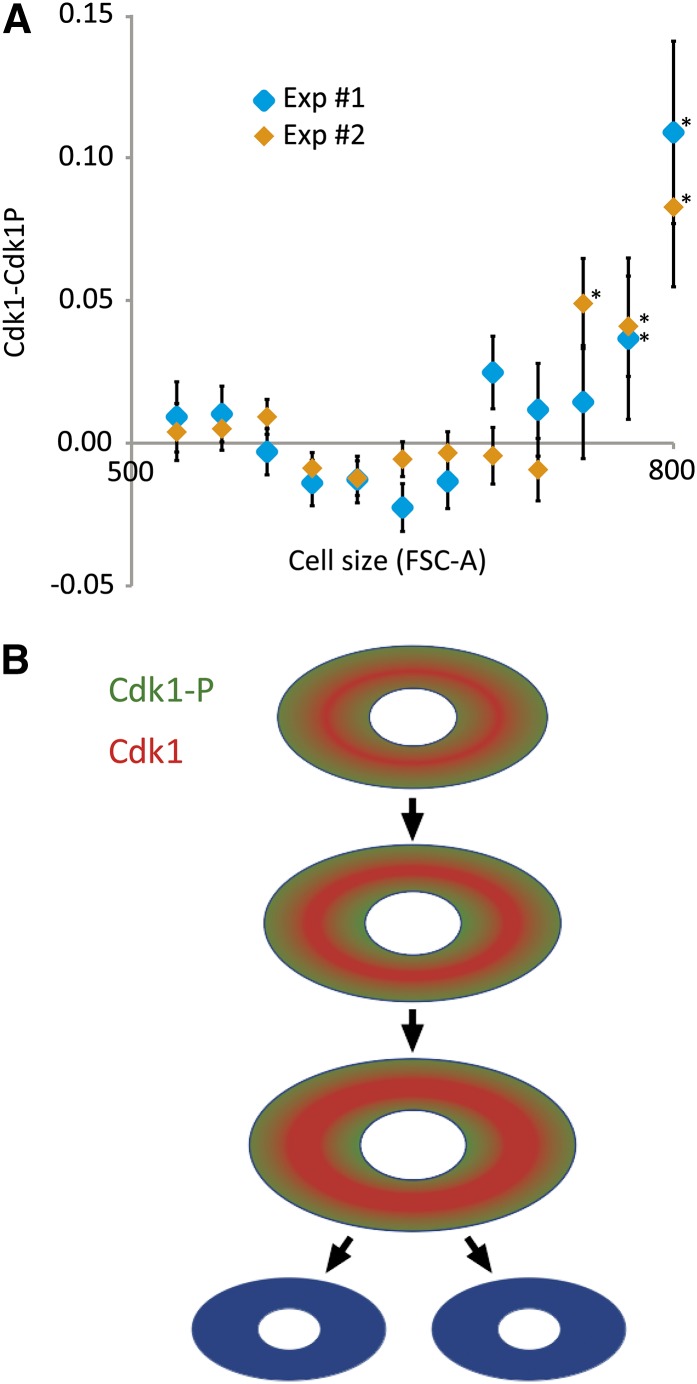
Figure 7 .
Regulation of cell size by Cdk1. (A) Relative amount of unphosphorylated Cdk1 (Cdk1-Cdk1P) in human U2OS osteosarcoma cells as a function of cell size (FSC-A). Two experiments using different sets of antibodies are shown. Note that unphosphorylated Cdk1 rapidly increases after the G2 cells reach a certain size. This non-linear increase is consistent with the fact that the Cdk1 inhibitory kinases Myt1 and Wee1 are localized in membranes and nucleus, respectively, whereas Cdk1 is soluble. Error bars indicate one standard error, and asterisks indicate P < 0.01 (Kolmogorov-Smirnov test). (B) Model of regulation of cell size. Different cells in a multicellular organism vary greatly in size, but sizes within a cell type are more constant. We propose that the constant cell size is maintained by the following mechanism: In small cells, Myt1 and Wee1 phosphorylate Cdk1 and convert it to Cdk1-P (green). When cells become larger, membrane bound Myt1 and nuclear Wee1 are unable to efficiently phosphorylate Cdk1, leading to cell size-dependent increase in Cdk1 activity (red) and entry into mitosis. In this model, setting the target size for different cell types requires an additional mechanism that regulates the concentrations of the soluble reactants (Cdk1, B-type cyclins and the string/Cdc25 phosphatase). Regulation of mRNA expression of both string/Cdc25 (Lehman et al. 1999; Grosshans and Wieschaus 2000) and Cdk1 (Lee et al. 1988; Lehner and O’Farrell 1990) has been described, and we show here that mRNA levels of Cdk1 and cyclinB correlate with cell size (see Figure 6, B and C).