Figure 3.
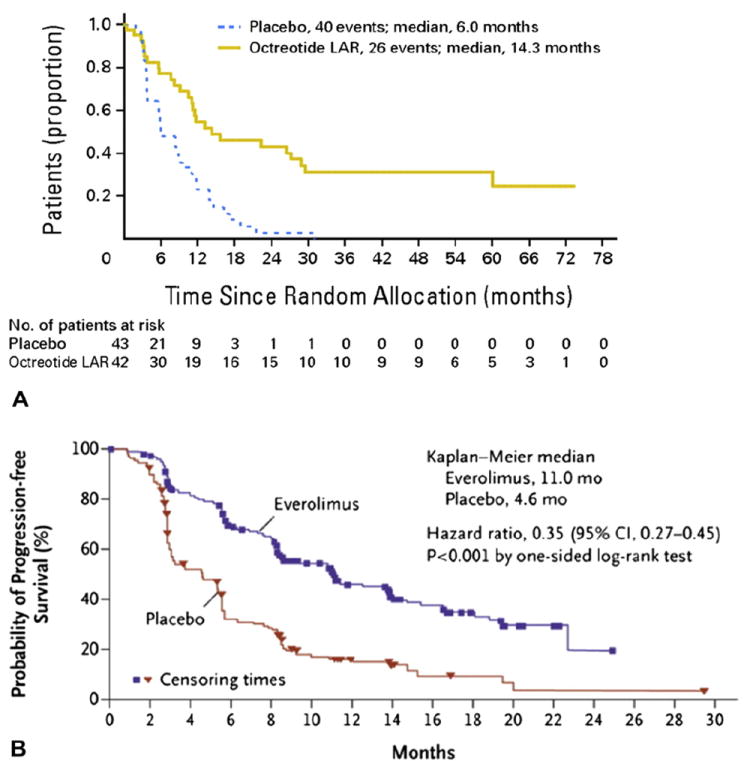
(A) Time to progression (TTP) in the Placebo-Controlled Prospective Randomized Study on the Antiproliferative Efficacy of Octreotide LAR in Patients with Metastatic Neuroendocrine Midgut Tumors trial for patients treated with octreotide LAR; the TTP in the octreotide LAR group was 14.3 months vs 6 months in the placebo group (p = 0.000072). Log-rank test stratified by functional activity: p = 0.000072; hazard ratio = 0.34 (95% CI, 0.20–0.59). (Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved. Rinke A, Müller HH, Schade-Brittinger C, et al; PROMID Study Group: Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol vol 27[28], 2009:4656–4663 [64].) (B) Progression-free survival (PFS) in the phase 3 RAD001 in Advanced Neuroendocrine Tumors (RADIANT-3) trial; patients in the everolimus group had a median PFS of 11.0 months compared with 4.6 months in the placebo group (p < 0.001). (From New England Journal of Medicine, Yao JC, Shah MH, Ito T; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group, Everolimus for advanced pancreatic neuroendocrine tumors, vol 364, pages 514–523. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.)
