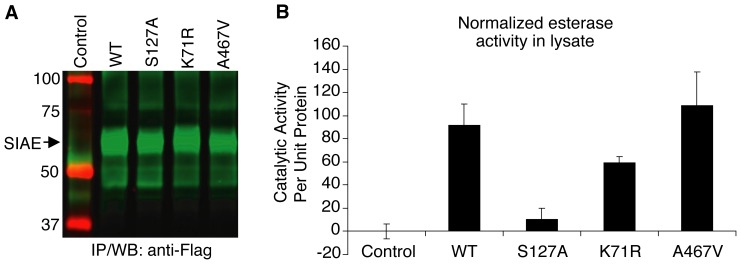
Figure 1. K71R SIAE and A467V SIAE proteins are functionally normal.
The K71R and A467V SIAE variants were re-created by site-directed mutagenesis. Wild type (WT) SIAE, a known catalytic site mutant (S127A SIAE), and the two SIAE common variants were transfected into HEK 293T cells. Half of each cell lysate was immunoprecipitated with anti-Flag antibodies and revealed in a quantitative Western blot assay (A) and the other half was similarly immunoprecipitated and examined for esterase activity, presented following normalization for lysate SIAE protein content (B). Each mutant was separately transfected and analyzed on three occasions. A representative experiment is shown. Error bars reflect esterase assays performed in triplicate in a single experiment.