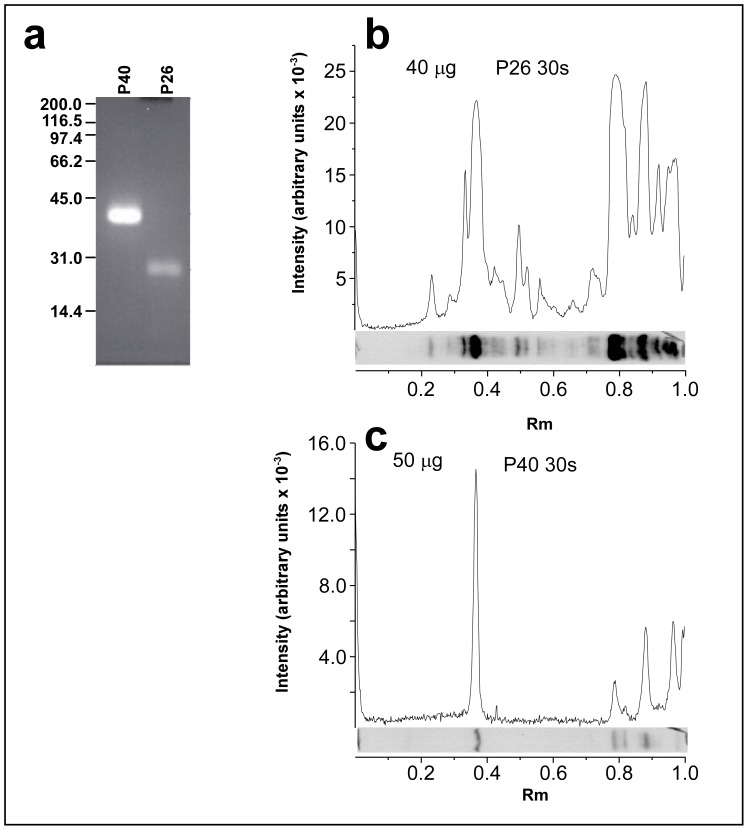
Figure 6. Ligand-blotting analysis of the interaction between recombinant polypeptides P40 and P26 with proteins from whole worm extracts.
Forty µg of recombinant P40 and P26 were labeled with fluorescein, subjected to SDS-PAGE and photographed under a short-UV lamp (a). Whole worm extract was subjected to SDS-PAGE (T = 10%) and transferred onto Hybond C membranes. Membrane strips containing the fractionated extract were incubated with fluorescein-labeled P40 (50 µg; 1125 pmoles)(b) and P26 (40 µg; 1520 pmoles) (c). After extensive washing, the bound recombinant fluorescein-labeled polypeptides were detected using an anti-fluorescein antibody labeled with peroxidase. The peroxidase activity was detected using chemiluminescence. The chemiluminescence reaction was exposed to Hyperfilm ECL (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) for 30 s in both experiments.